Introduction
Dr. Max Ellenberg, who dedicated his life to the clinical care for patients with diabetic neuropathy in the mid 20th century, concluded that neuropathy is not a complication of diabetes but a concomitant with the same . This foresight has been substantiated by the following clinical evidence that (1) neuropathy may be the initial manifestation of diabetes, (2) neuropathy is the most prevalent among individuals with comorbidities or various complications, (3) neuropathy is often present in prediabetic stage, (4) neuropathy occurs in the absence of overt hyperglycemia or during good control, (5) signs and symptoms of neuropathy are not parallel with severity of diabetes or duration of diabetes. Despite the advancement in knowledge concerning diabetic neuropathy, its complexity continues to be highly perplexing. In February 1988, San Antonio consensus panel announced that “diabetic neuropathy is a descriptive term meaning of a demonstrable disorder, either clinically evident or subclinical, that occurs in the setting of diabetes without other causes for peripheral neuropathy” . This definition was succeeded by subsequent panel conferences, and the entity of neuropathy includes all manifestations in the somatic and/or autonomic parts of the peripheral nervous system . Hence neuropathy may not simply be “one of the complications” but is likely to represent diabetes itself.
Patients with neuropathy suffer from intractable pain, uncomfortable sensation, severe autonomic imbalance, and lowered quality of life. Nearly half of the patients with diabetes are affected with such neuropathic complications and often require expensive limb amputation . Care for neuropathy is critical to the management of diabetes not only because of its intractable symptoms but also of serious impact on life expectancy . With longer duration of diabetes, the incidence of neuropathy is increased . Neuropathy manifests early in prediabetic stage in type 2 diabetes (T2D). Because of the insidious nature of its symptoms, accurate pathologic features in early neuropathy are not well established, although involvement of small nerve fibers has recently been proposed. Currently, there is no effective means for the prevention and suppression of the development of neuropathy except for meticulous blood glucose control. For a better understanding of the pathophysiology of diabetic neuropathy and determination of the treatment design, it will be useful to review the pathologic basis of diabetic neuropathy.
Classification of diabetic neuropathy
Diabetic neuropathy is a complex disorder with metabolic and vascular basis. Long-term hyperglycemia or hyperlipidemia exerts chronic injurious and reparative remodeling of the peripheral nerve tissues. In this setting, degenerative, regenerative, inflammatory lesions develop diffusely in centripetal manner on the basis of metabolic aberration . In contrast to metabolic deficits, compromised vascular supply triggers acute infarction or chronic ischemic changes, resulting in mainly focal or multifocal pathological phenotype . Thomas divided diabetic neuropathy into two major types of diffuse and focal neuropathy ( Table 2.1 ). Diffuse type represents distal symmetric sensory predominant neuropathy. This type is commonly accompanied by involvement of autonomic nervous system of both sympathetic and parasympathetic nerves . The presence of cardiac autonomic neuropathy markedly reduces the quality of life and shortens life expectancy . Distal sensory–predominant neuropathy usually accompanies autonomic neuropathy, and this combination is collectively called diabetic polyneuropathy (DPN).
|
Focal type may be subclassified into mononeuropathy and multiple mononeuropathies (mononeuropathy multiplex). Mononeuropathy is typical for cranial neuropathies (oculomotor nerve, abducens nerve, etc.) presenting ophthalmoplegia . Less clearly differentiated are truncal neuropathy and proximal motor neuropathy of the arm or the thigh (diabetic amyotrophy) from multiple mononeuropathies. The latter is now regarded as lumbosacral radiculoplexus neuropathy (LSRPN) . Mode of the onset may be divided into acute, subacute, or chronic, the former of which is atypical compared to chronically developed DPN. Motor type of cranial neuropathy occurs as sudden or rapid onset of painful oculomotor palsy . In contrast, proximal motor neuropathy manifests acute or subacute focal painful muscle atrophy in the arm, thigh, or thorax . In cases with obvious muscle atrophy in extremities or trunk, it was used to have the term of diabetic amyotrophy . This terminology is not used nowadays because it does not indicate the accurate causation. Proximal motor neuropathy is associated with epineurial vasculitis with a pattern of multiple mononeuropathies . In this case, neurologic deficits involve proximal radiculoplexus neuropathy with motor deficits. The association of vasculitis with muscle atrophy suggests immunogenic basis in its pathogenesis . There remain some ambiguities and complexities for the clinical phenotype and their backgrounds. Nevertheless, efforts for the separation of clinical phenotype of neuropathy are meaningful and important for the understanding of the disease and for the decision of treatment design.
Basic pathologic changes in several types of neuropathy
Pathological basis of DPN appears to be distinct in each type of neuropathy. There may be some overlap or transient form of the disorder. For the convenience of understanding, representative features will briefly be introduced in each type, and the implication of the lesions related to the clinical phenotype will be discussed.
Classical pathological studies on diffuse type of DPN were conducted on the materials obtained at the time of autopsy . Most conspicuous changes were detected in the peripheral nerve showing loss of nerve fibers, demyelination/remyelination and fibrosis. The changes were more robust in the dorsal root compared to ventral root, suggesting sensory predominance of the lesion . Dorsal column underwent demyelination and gliosis, but there were not any substantial changes in dorsal root ganglia . Sympathetic and parasympathetic nerves contained nerve fibers with degeneration and demyelination . Ganglion cells in celiac and mesenteric ganglia were vacuolated and markedly swollen . Autonomic nerve alterations supplying the internal organs also indicated the long-term presence of structural derangement in autopsy cases of diabetic patients . Despite the presence of apparent pathological changes such as neuronal loss and reactive gliosis in the spinal cord or dorsal root ganglia, it was not clear whether the changes really represented the alterations caused by diabetes or other factors because of the lack of precise clinical information. Common appearance of neuromatous changes showing bundle of proliferative nerve fasciculi in gastrointestinal walls or vascular walls suggested the repeated injury to internal autonomic nerves and its reactive growth resembling traumatic neuroma . Most of the changes were not specific for diabetes but merely reactive process to chronic injury.
Later systematic investigations of autopsy materials embedded in epon after fixation with glutaraldehyde and osmium provided more precise spatial distribution of nerve lesions in the peripheral nervous system. On the semithin cross-sections of the sciatic, tibial, and sural nerves, nerve fiber loss with thickened endoneurial vascular walls was consistently demonstrated . The results from these studies were informative, but the subjects investigated were limited and often complicated with uremia, and therefore causal relationships of nerve lesions with clinical phenotype were not well addressed . Such systematic studies are extremely valuable but too laborious to collect the data from sufficient number of subjects to draw an appropriate conclusion.
Following the autopsy studies, investigations on the biopsied sural nerve samples from patients with clinical information on neuropathy were commenced at the light and electron microscopic levels. To this end, freshly obtained sural nerve tissues were applied to teased nerve fiber studies and light microscopic observations on the semithin–thick epon sections stained with toluidine blue or phenylenediamine . The ultrathin sections were investigated using electron microscope after uranyl acetate staining. Teased fiber method was developed for the identification of myelinated fibers for axonal degeneration, demyelination, and remyelination . Semithin sections were suited for the morphometric analysis of myelinated fibers , whereas unmyelinated nerve fibers and fine subcellular changes were depicted only by electron microscopic observations . Despite considerable information obtained from the biopsied samples, this method is not recommended any more in clinical practice because of the invasiveness and postsurgical complication of sensory loss in the lateral side of the foot, except for the exceptional occasion of necessity for differential diagnosis or special research purpose . Alternative site of biopsy with less troublesome complications was also introduced in case of the necessity of structural analysis of the peripheral nerve .
Currently, less invasive methods for pathological investigations on the skin biopsy have evolved as a standard for detection of neuropathy in diabetes . Immunohistochemical staining with antibodies against protein G product 9.5 (PGP9.5) as well as the use of markers for basement membranes or microvessels enable the evaluation of small nerve fibers (C-fibers in the epidermis and of Aδ fibers in the dermis). With the advent of this method, early reduction of intraepidermal nerve fiber density (IENFD) as an index of neuropathy was confirmed in subjects with prediabetes .
More recently, corneal confocal microscopic (CCM) observations on the cornea have developed for the detection of small nerve fiber abnormalities in patients with diabetes. This method is not invasive and feasible for repeated investigations . The topic on CCM will be precisely described in another chapter in this book ( Chapter 4 ). General outline of pathological characteristics will be described in each type of neuropathy in the following sections.
Sensory nerve pathology
Distal nerve fiber loss
Salient feature of the peripheral nerve in DPN is nerve fiber loss with distal predominance ( Fig. 2.1 ). Remnant fibers also undergo axonal degeneration and regeneration, segmental demyelination, and remyelination. With regard to the length, distal sensory sural nerve originating from the sciatic nerve usually displays the most severe changes . Deficit of regenerative capacity accelerates fiber loss in diabetes.
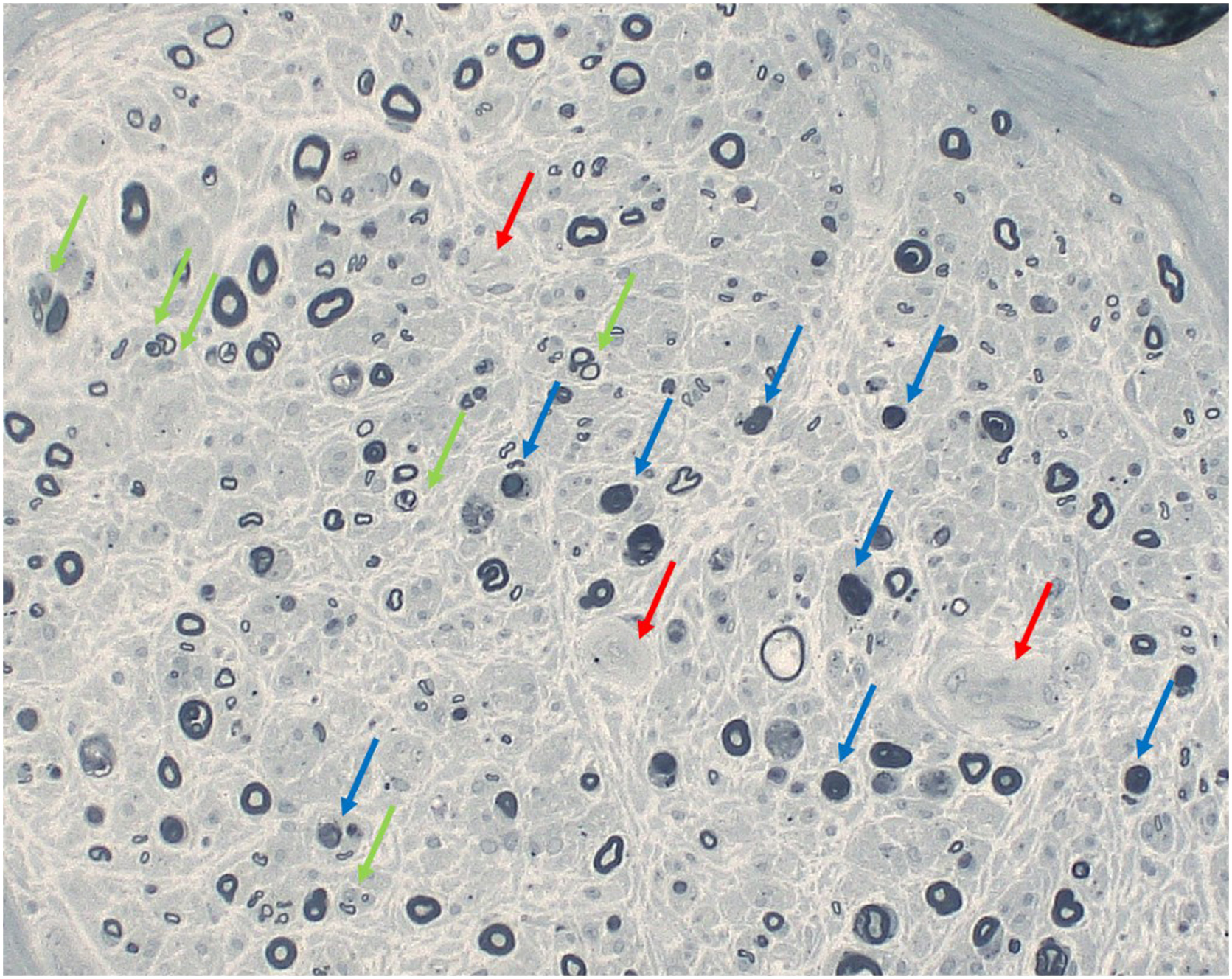
Thomas first attempted to connect the pathologic basis with the clinical signs and measures of neuropathy. He concluded that the fiber changes did not well explain the clinical signs and symptoms but well corresponded to the conduction delay. Behse et al. provided a good correlation of myelinated fiber loss in the biopsied sural nerve with the severity of nerve conduction deficits. Loss of nerve fibers was not limited to specific population of nerve fibers but involved large and small myelinated and unmyelinated fibers referred to as panmodal nerve fiber loss. They considered that 20%–30% of the conduction delay was not explained by the nerve fiber loss. Teased fiber studies showed predominantly segmental demyelination and remyelination , once led to the hypothesis that diabetic neuropathy is Schwann cell disease. With advancement of nerve physiology technology, fiber loss well corresponded with the reduction of evoked amplitudes (sensory nerve action potentials, i.e., SNAP or compound muscle action potentials, i.e., CMAP) in diabetic patients with overt neuropathy . It was shown that even under good blood glucose control there occurred progressive decline of CMAP or SNAP over 4–7 years of observation period . There was no significant correlation, however, between measures of nerve fiber size, g-ratio (axon/fiber size ratio), electrophysiologic parameters, and clinical indices of sensory tests or painful symptoms .
The degree of fiber loss became more pronounced with advancing stage of neuropathy . The extent of nerve fiber loss was quantitatively expressed by the reduction of the myelinated nerve fiber density (MNFD), which represented the number of myelinated nerve fibers within the unit area of each fascicle expressed as #/mm 2 . This measure well correlated with clinical staging of diabetic neuropathy . The nerve fiber loss appeared to clinically manifest with the appearance of negative symptoms such as loss of protective sensation or numbness . In contrast, positive subjective symptoms such as pain or paresthesia triggered by the signals released from stimulated fibers, or spinal cord, or brain in chronic state, appeared at any time when the impulse was evoked from remnant nerve fibers or even in the absence of the nerve fibers (phantom pain) ( Fig. 2.2 ). The nerve pathology depicted so far, however, is only cross-sectional in nature, and therefore no sequential information is available regarding the way of progression.
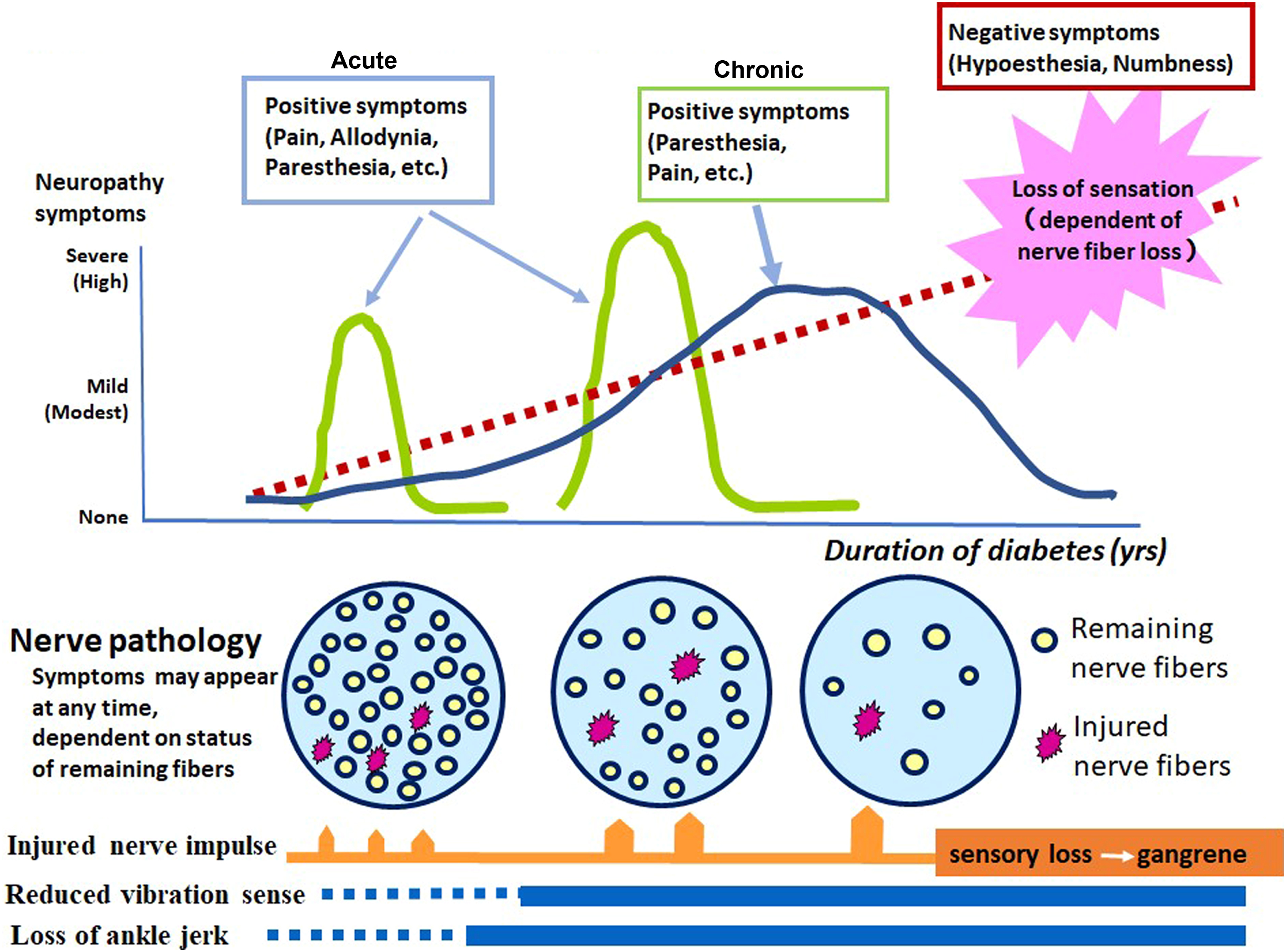
There are some exceptional studies employing repeated sural nerve biopsy to explore the treatment effects on DPN by measuring MNFD prior to and after the trial . Without treatment, there was approximately 1%–2% decline of MNFD after 52 weeks in diabetic subjects. Since percentage change of MNFD (ΔMNFD) included lost nerve fibers (5%–6%) along with regenerating fibers (4%–5%), set off number of changed nerve fibers among remaining fibers was approximately 1%–2% either increased or decreased (which is equal to the sum of the number of regenerating nerve fibers − lost myelinated nerve fibers). As results, they demonstrated significant suppressive effects of progressive MNFD decline in diabetic patients treated with aldose reductase inhibitor (ARI) sorbinil compared to placebo group . In those studies, they found 3.8 times increase in the fraction of regenerated nerve fibers with 33% increase in total. It is thus conceivable that the effects of sorbinil were mainly the promotion of the nerve fiber regeneration . Unfortunately, sorbinil was dropped out from the market because of serious adverse effects. In contrast, another ARI (zenarestat) treatment did not exhibit any significant increase in MNFD with no difference between treated and untreated group . Since precise analysis of nerve fiber caliber spectrum was not presented, it is not clear whether small regenerating fibers were in fact increased or not or whether loss of large fibers was protected.
Nerve biopsies are too invasive merely for the evaluation of treatment effects and are thus not recommended nowadays. However, recent microarray or proteomics analyses on the stored samples obtained from clinical trials provided valuable information on the question whether there are responders or nonresponders or how progressive fiber loss is related to genetic, inflammatory, or metabolic factors . Details of the findings are reported in the literature, but the relationships of genes and molecules to pathological features are yet to be explored.
Metabolic and vascular basis for nerve fiber loss and heterogeneity of neuropathy
In contrast to generalized loss of myelinated fibers in distal sural nerve, the spatial distribution of focal nerve fiber loss in the proximal sciatic nerve suggested a vascular basis for diabetic neuropathy . The focality of the lesions was more prominent in the proximal nerve but less obvious in the distal side . Such distribution of nerve fiber loss was postulated to be ascribed to the occlusive or obstructive lesions of nutrient vasa nervorum . It was also speculated that such distribution reflected ischemic lesions of myelinated fibers undergoing axonal degeneration in the proximal site and summation of the focality became gradually diffuse fiber loss in the distal portion ( Fig. 2.3 ). To confirm this possibility, peripheral nervous system was examined in a rat model of microsphere infarction, which displayed vasculitic neuropathy, showing spatial distribution of nerve lesions, similar to those depicted in diabetic patients . By means of a series of experiments and observations, the researchers concluded that fiber loss was primary and vascular in origin, and ischemia/hypoxia was a cause of diabetic neuropathy .
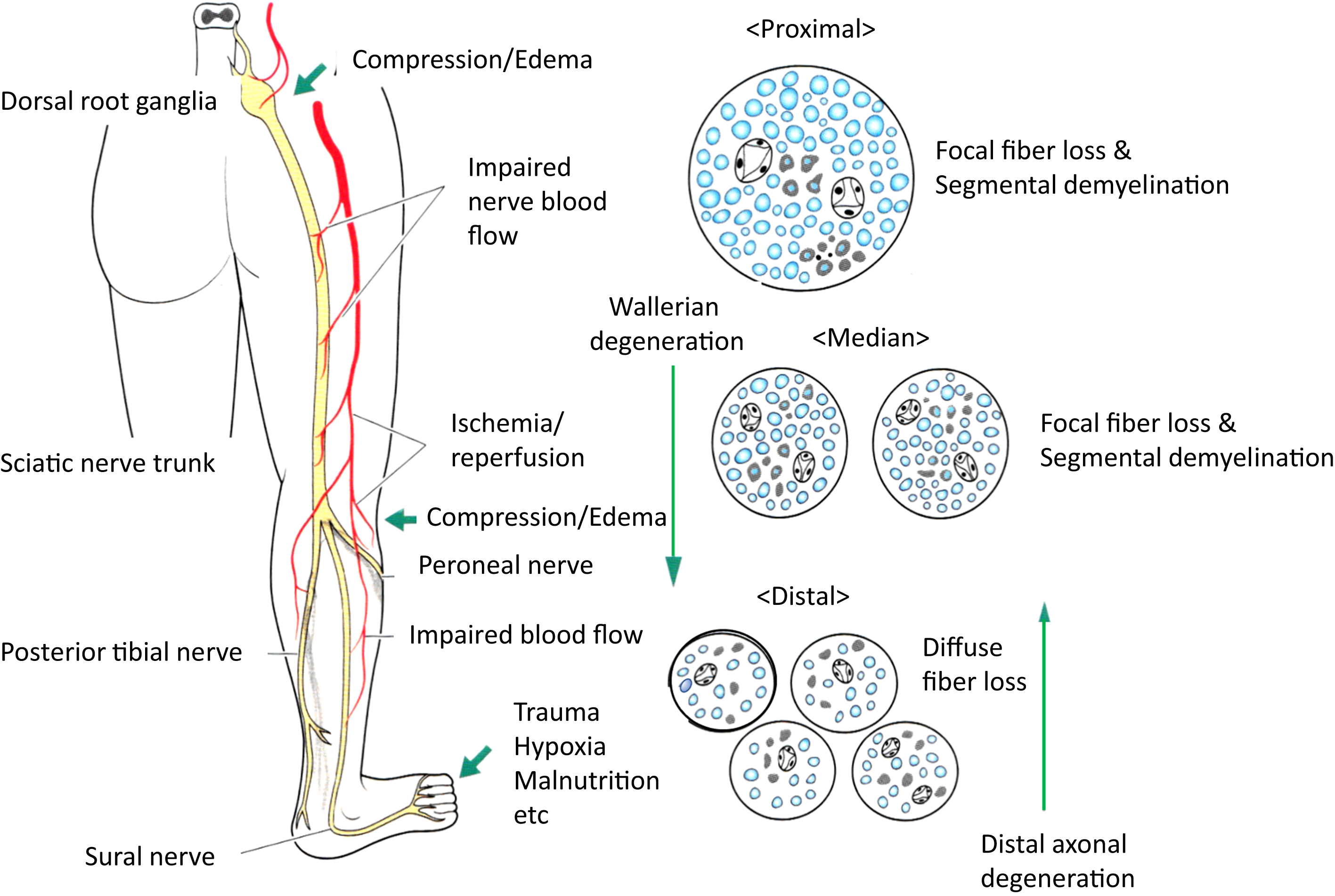
There were some disagreements against the vascular hypothesis derived from the focality of fiber loss lesions in diabetic neuropathy. Thomas and his colleagues described that the patchy distribution of fiber loss was comparably found in a series of sural nerve biopsies in type 1 hereditary motor and sensory neuropathy (Charcot–Marie–Tooth disease), which had a genetic basis free from vascular influences, thereby questioning the vascular causation of diabetic neuropathy . To reconcile this discrepancy, it was interpreted that Dyck et al. examined mostly older subjects much influenced with vascular alterations , whereas Thomas and his group examined mainly younger subjects with type 1 diabetes (T1D) . Alternatively, the pattern of fiber loss is likely to be different between T1D and T2D, showing nonuniform pathological features in diabetes. Sima and their colleagues demonstrated significant differences in the distribution of nerve fiber loss in the sural nerve between T1D and T2D when subjects were adjusted to comparable age, duration of diabetes, severity of hyperglycemia . In the study, focality of fiber loss was determined by the coefficient variation (CV) of MNFD from area to area of nerve fasciculi, and there was a significantly greater CV in T2D than that in T1D. They concluded that the focal nerve fiber loss was considered to have a vascular origin in T2D, whereas the changes in T1D were diffuse, suggesting metabolic origin ( Table 2.2 ). It is worth noting that not limited to hyperglycemia, hyperlipidemia may be invoked in the development of neuropathy in T2D . To confirm the implication of hyperglycemia, meticulous blood glucose control effectively inhibited the progression of neuropathy in Diabetes Control and Complication Trial (DCCT) study . In contrast to this result, failure of tight blood glucose control for the suppression of neuropathy progression observed in patients with T2D in UK Prospective Diabetes Study group investigation indicated the difference in the pathogenetic processes of neuropathy between T1D and T2D. Differences in neuropathy between T1D and T2D are still speculative, however, and needs to be clarified by future investigations .
| Nerve fiber | Vascular | |||||||
|---|---|---|---|---|---|---|---|---|
| Major cause | Fiber loss | Pattern b | Atrophy | Axoglial contact c | Endothel | Cell junction d | Basement membrane | |
| T1D | Metabolic | diffuse | Axonal degeneration | Yes | Impaired | Nonoccluded | Cohesive | Mildly thickened |
| T2D | Vascular | Focal | Wallerian degeneration | No | Equivocal | Occluded | Separated | Thick and multiplicated |
a Differences in the structural changes between type 1 (T1D) and type 2 diabetes (T2D) proposed by Sima et al. [73].
b Pattern; major type of nerve fiber degeneration.
c Putative structure of axoglial dysjunction proposed by Sima et al. [73].
d Contact between two endothelial cells with tight junctions by Sima et al. [128].
Demyelination and ultrastructure of Schwann cells
It is worth noting that fiber loss was not always evident as an early pathological feature in diabetic neuropathy. Not in frequently, demyelination and remyelination were commonly encountered in teased fiber studies . Thomas and Lascelles reported frequent appearance of fibers undergoing segmental demyelination in addition to the loss of nerve fibers and Schwann cell proliferation in biopsied radial nerve from eight cases with diabetes. Chronic injury to Schwann cells was suspected to be mainly implicated in the diabetic nerve pathology. Further investigation on sural nerves obtained from 19 relatively young diabetic patients (age: 20–<60 years) with painful and autonomic neuropathy showed marked loss of large and small nerve fibers accompanied by fibers with axonal regeneration and remyelination . They found difficulty to connect the pathological findings to clinical symptoms. Said et al. studied sural nerve pathology from five young (age: 20–<30 years) subjects with T1D with severe early onset neuropathy . They found 20% reduction of myelinated nerve fibers, and some with dying-back type, accompanied by 33% fibers of segmental demyelination and 11% axonal degeneration. Often, regenerative and remyelinated fibers occupied considerable percentage area of nerve fasciculi . Such de- and remyelination changes may probably be secondary to axonal degeneration because distal to the site of axonal degeneration, segmental demyelination ensues. It may also be possible that the changes of myelin sheath occur primarily in face of increased endoneurial pressure or compression effects because endoneurial edema is common in diabetic peripheral nerve .
Using electron microscopy (EM), axonal degeneration was characterized by depletion of axonal organelles such as neurofilaments, microtubules, and smooth endoplasmic reticulum (ER), as well as mitochondria ( Fig. 2.4 ). Oftentimes, there appeared concurrent aggregation of glycogen granules, autophagic vacuoles, and phagosomes, as well as engulfed myelin debris. Glycogen accumulation enclosed by limited membrane was considered to be a sign of hypoxic changes . Structural changes of Schwann cells also assumed a variety of patterns showing degenerative, reactive, regenerative, and proliferative changes . Invagination of Schwann cell cytoplasm into the axolemma and disruption or delamination of myelin lamellae were also found. Such disruption of myelin structure probably reflected the process of demyelination . Schwann cell cytoplasm contained degradation products of disrupted myelin fragments or autophagic vacuoles. Occasionally, multilayered Schwann cell cytoplasmic processes surrounded regenerated nerve axons, showing onion–bulb appearances, often called hypertrophic changes. The regenerative Schwann cells accompanied thickened and elongated projections of basement membranes , which appeared to result from the renewal or replicated Schwann cells containing several small regenerating axons ( Fig. 2.4 ). Vacuolar cytoplasmic organelles are often misinterpreted as pathological signs, but they may frequently be artifacts during the process of fixation. Most of the changes detected by EM were not specific nor pathognomonic, simply expressed the pathological signs. Variety of axonal disintegration represented multiple facets of ischemic nerve injury . No causal relationships were obtained for subcellular changes, not indicative of etiology.
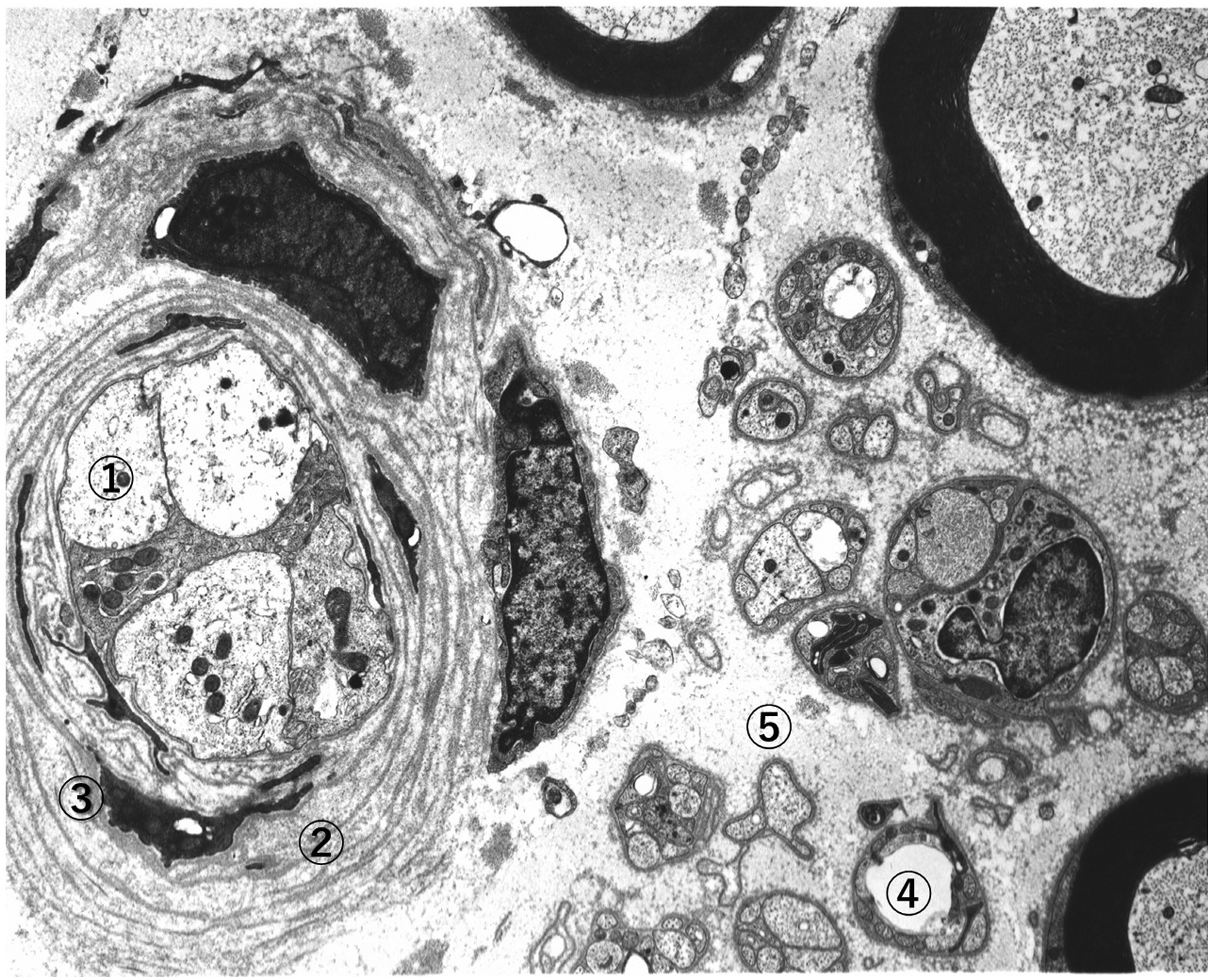
Sural nerve biopsy is not routinely conducted any more for the purpose of diagnosis, but on the special occasion, nerve biopsy is still required for the diagnosis and decision of treatment. When differential diagnosis from amyloidosis, chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), and Charcot–Marie–Tooth diseases is required, special diagnostic procedures such as immunostaining and genetic analyses may be needed on the tissue sections .
Impaired regeneration and related factors
Progressive nerve fiber loss in diabetes may also be attributed to impaired regeneration of nerve fibers. Evaluation of density of regenerative nerve fiber cluster was undertaken to estimate the balance between degeneration and regeneration of myelinated fibers . In a sural nerve biopsy study, the number of regenerating fibers, identified using light microscopy, was found to decline in proportion to the reduction in total MNFD. In contrast, number of regenerating fibers relative to total myelinated fibers was significantly greater in patients with T1D compared to T2D . This may be accounted for by younger age population in T1D compared to T2D, while regenerative capacity is less potent in T2D. On the other hand, there was no significant correlation between the number of regenerative clusters and painful symptoms .
Recently, Feldman’s group studied the genetic role in the impaired regeneration of nerve fibers in DPN. Hur et al. identified risk factors associated with sural nerve fiber loss in patients with diabetic neuropathy. In their study, diabetic patients with neuropathy treated with acetyl-L-carnitine for 52 weeks were retrospectively examined. Sural nerve biopsy was performed before and after the trial . In accordance with the change of myelinated fiber density (ΔMFD%), subjects were divided into three groups of regenerator, degenerator, and intermediate, with dramatically increased, decreased, and steady ΔMFD%, respectively. HbA1c at baseline was the only factor significantly different between regenerator and degenerator (OR 0.68; P <.01). Microarray analysis revealed that upregulated genes in the regenerator group were enriched with cell cycle and myelin sheath function, while downregulated genes were enriched in immune/inflammatory responses . These data suggest that HbA1c levels predict myelinated nerve fiber regeneration and degeneration in patients with DN.
Motor nerve pathology
Large fiber dysfunction is a constant feature of DPN. Previous studies disclosed degenerative changes and nerve fiber loss in the peripheral motor nerve . Indeed, patients with long-term diabetes or sustained metabolic aberration usually displayed delayed nerve conduction and lowered action potentials in both sensory sural nerve and motor tibial nerve . The severity of DPN was in fact represented by the lowered action potentials of CMAP which corresponded with the extent of reduced nerve fiber density . Consistent with the changes of sensory nerves, motor nerves of sciatic nerve or tibial nerve showed fibers undergoing active axonal degeneration, segmental demyelination and fiber loss . The motor nerve involvement in DPN may be reflected by the presence of muscle atrophy with distal predominance . Degenerative changes of motor nerves resulted in progressive muscle atrophy. Motor unit denervation of muscle and decreased muscle cell size and diminished capillaries were also described . Instability, gait disturbances, and muscle weakness were major clinical signs of motor nerve involvement. In the classic literature, motor nerve endings in contact with muscles often underwent denervation and synaptic degeneration . Disruption of muscle spindles and intrafusal fibers were also reported . Autopsy-based study disclosed the presence of central chromatolytic neurons in the anterior horn and reactive gliosis, suggesting retrograde nerve fiber degeneration . It is not still clear, however, whether the changes were primarily induced by diabetes or were secondary to the wasting of muscle tissues.
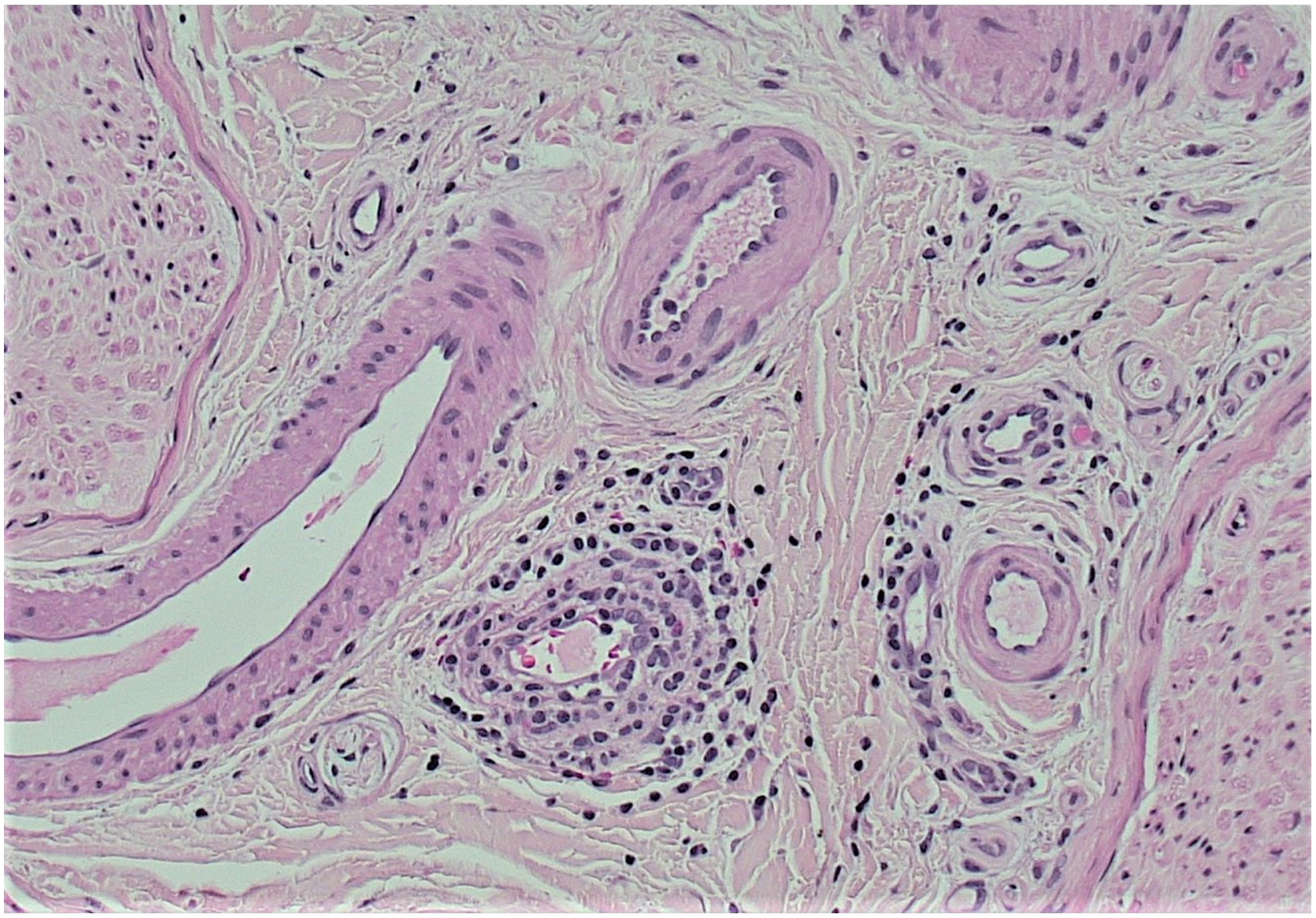
Motor predominance of neuropathy may be characteristic in some variants of DPN, such as mononeuropathy or multiple mononeuropathies. Neuropathy with motor predominance should strictly be differentiated from sensory-predominant type of DPN because treatment option is different. Mononeuropathy was typically characterized by sudden onset of painful ophthalmoplegia in cases of cranial neuropathy, which usually resolved spontaneously without any treatment . Thrombotic occlusion of nutrient arteries was considered to be the cause by histological observations on the affected nerve.
Motor predominant neuropathy with painful muscle atrophy occurs in elderly people with or without diabetes. In cases with diabetes, this condition was used to be called as diabetic proximal motor neuropathy or diabetic amyotrophy. Currently, this condition is called diabetic lumbosacral radiculoplexus neuropathy (DLSRPN) . Biopsy studies disclosed frequent association of microvasculitis in these patients . Said et al. documented the presence of vasculitis around perineurium in 10 cases with T2D and painful proximal neuropathy with muscle atrophy. With vasculitic lesions, peripheral nerves showed nerve fiber loss with features of axonal degeneration and myelin breakdown, indicative ischemic effects on the nerve ( Fig. 2.5 ). In their subsequent studies on the diabetic patients with painful multifocal neuropathy, they found marked reduction of nerve fiber density, associated with a necrotizing vasculitis of perineurial and endoneurial blood vessels in the root, plexus, and nerve trunk, further supporting the immunogenic basis of this disorder . Similar findings were detected by Dyck’s laboratory collecting a relatively large cohort of patients and also reported by other group . Painless cases were also encountered .