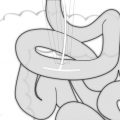
Fig. 6.1
Precursor lesion of intestinal type gastric cancer – dysplasia. (a) Low-grade dysplasia. Pseudostratification of nuclei. Nuclei are elongated and mostly basally orientated. Few mitotic figures. (b) High-grade dysplasia. Crowding of nuclei. Nuclei are larger and rounder and vary more in size and shape. Loss of basal orientation of nuclei in many cells. Basal membrane around individual glands still intact
Low-grade dysplasia progresses to adenocarcinoma in up 23 % of cases within 10 months to 4 years, whereas malignant transformation of high-grade dysplasia has been reported to occur in 60–80 % of cases.
It is noteworthy that precursor lesions of diffuse type GC are not well characterized, except for hereditary diffuse type GC (see below under the section on genetic predisposition and hereditary syndromes).
Pathology of Gastric Adenocarcinoma
Macroscopy
GC can present at an early or advanced disease stage. “Early gastric carcinoma” (EGC) is defined as a carcinoma which has infiltrated the mucosa or submucosa regardless of the presence or absence of lymph node metastases [19, 20]. Conversely, GCs infiltrating into the muscularis propria and beyond are defined as “advanced.”
EGCs are classified into three types based on the endoscopic appearance according to the Paris classification: type I (protruded), polypoid growth (subcategorized into Ip (pedunculated) and Is (sessile)); type II (superficial), non-polypoid growth (subcategorized into type IIa (slightly elevated), type IIb (flat), and type IIc (slightly depressed)); and type III, excavated growth [21] (Figs. 6.2 and 6.3).
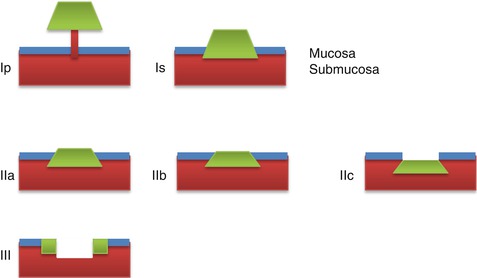
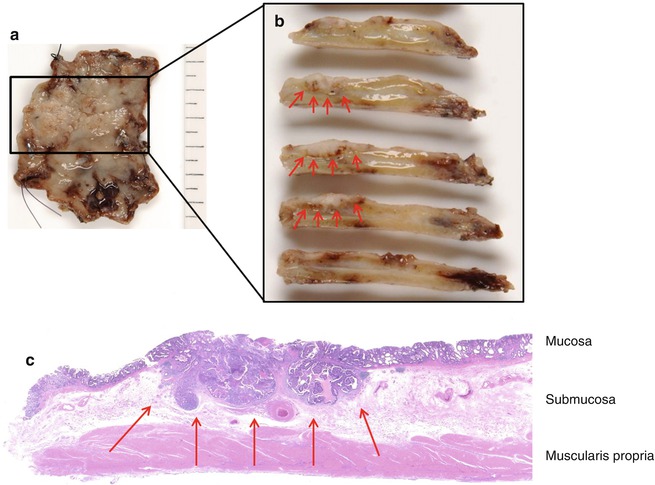

Fig. 6.2
Paris classification of early gastric cancer: type I (protruding), Ip pedunculated and Is sessile; type II (superficial), IIa elevated, IIb flat, and IIc slightly depressed; type III (ulcerated)

Fig. 6.3
Endoscopic resection (ESD) of a well-differentiated early gastric cancer. (a) Macroscopy of the endoscopic resection specimen after fixation with a superficially elevated lesion (Paris type IIa). (b) Macroscopy of the serial cross sections through the lesion showing a tumor which is infiltrating the submucosa (red arrows). Deep resection margin located in the muscularis propria ensuring complete (curative) resection of the tumor. (c) Microscopy of the well-differentiated adenocarcinoma infiltrating the submucosa (pT1b, red arrow) and adjacent intramucosal adenocarcinoma (Images courtesy of Dr. T. Arai, Tokyo)
The macroscopic appearance of advanced GC is classified using the Borrmann classification [22] which divides GC into four distinct types: type I, polypoid type; type II, fungating; type III, ulcerated; and type IV, diffusely infiltrative (Fig. 6.4).
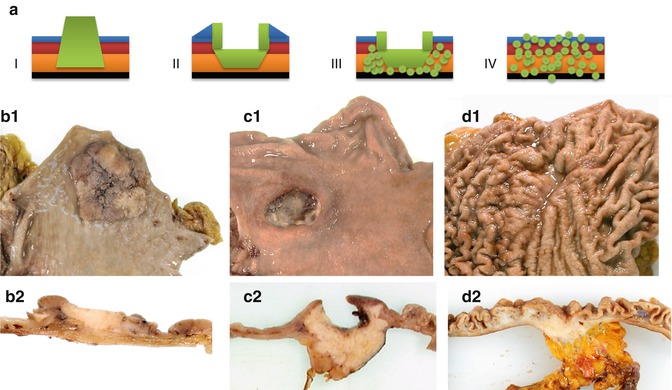

Fig. 6.4
Macroscopy of advanced gastric cancer. (a) Borrmann classification. I polypoid type, II fungating type, III ulcerated type, and IV diffusely infiltrative. (b) Polypoid gastric cancer (type I) – (b1) macroscopy of the mucosal surface showing a large polypoid lesion and (b2) cross section showing tumor infiltrating into the superficial layer of the muscularis propria. (c) Ulcerated gastric cancer (type III) – (c1) deep ulceration visible macroscopically from the mucosal surface and (c2) infiltration into the attached lesser omentum visible macroscopically on cross sectioning. (d) Diffusely infiltrative gastric cancer (type IV) – (d1) diffuse thickening of the gastric folds visible on macroscopy of the mucosal surface and (d2) diffuse infiltration of the whole depth of the wall into the perigastric fat on cross section
Microscopy
While the macroscopic appearances are different between early and advanced GC, the histological appearances are similar. Two major histological GC subtypes (intestinal type GC and diffuse type GC) have been described by Laurén [23] which have different clinicopathological profiles and molecular pathogenesis and often occur in distinct epidemiologic settings.
According to the World Health Organization (WHO) [1], GCs are classified as tubular, papillary, mucinous, poorly cohesive (with or without signet ring cells), and mixed (Fig. 6.5). Tubular and papillary carcinomas roughly correspond to the intestinal type and poorly cohesive carcinomas correspond to the diffuse type according to Laurén’s classification (Table 6.1). The Laurén and WHO classifications are the ones most commonly used outside of Japan. In Japan, the recommended histological typing is similar but not 100 % identical to the WHO classification [24].
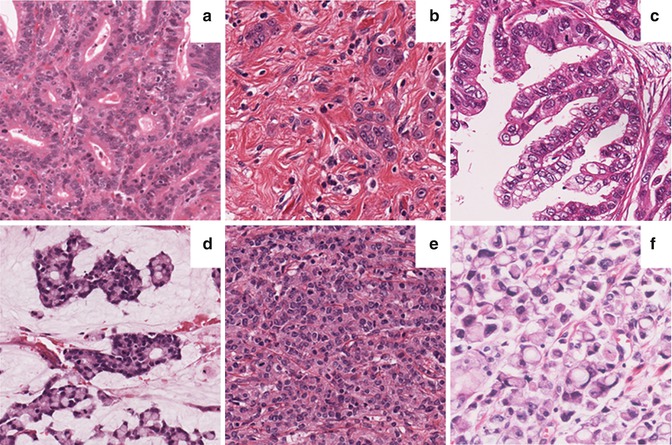

Fig. 6.5
Histological subtypes of gastric cancer. (a) Tubular type (moderately differentiated); (b) diffuse type; (c) papillary type; (d) mucinous type; (e) undifferentiated, solid type; and (f) poorly cohesive type with signet ring cells
Table 6.1
Classification of GC
Laurénclassification | World Health Organization 2010 | Japanese classification 2011 | Nakamuraclassification |
|---|---|---|---|
Intestinal type | Papillary | Papillary | Differentiated type |
Tubular | Tubular 1 | ||
Tubular 2 | Undifferentiated type | ||
Mucinous | Mucinous | ||
Diffuse type | Poorly cohesive, includingsignet ring cell carcinomaand other variants | Signet ring cell | |
Poorly differentiated, non-solid type | |||
Mixed (intestinaland diffuse type) | Mixed type (tubular/papillary and poorly cohesive/signet ring) | – | – |
Indeterminate type | Undifferentiated | Poorly differentiated, solid type | Undifferentiated type |
Adenosquamous | |||
Medullary | |||
Hepatoid |
Nakamura’s classification into differentiated and undifferentiated subtype is used together with the size of the lesion and presence or absence of ulceration to decide whether a lesion can be treated endoscopically [25, 26]. Apart from the classifications based on tumor morphology, GC can be classified on the basis of the presence or absence of cell differentiation markers – MUC5AC and trefoil peptide TFF1 (markers of surface gastric epithelium (foveolar cells)), MUC6 and trefoil peptide TFF2 (markers of mucus neck cell, pyloric gland, and Brunner’s gland cells), and MUC2, CDX-2, and CD10 (markers of intestinal goblet cells) – into four phenotypes: (1) gastric, (2) mixed gastric and intestinal, (3) intestinal, and (4) unclassifiable or null phenotype which does not express any of these markers [27–29].
Staging and Prognosis of Advanced Gastric Cancer
Staging
The staging for carcinoma of the stomach was substantially modified in 2009 as detailed in Table 6.2. Major changes included the subdivision of T1 cancers into T1a (mucosa) and T1b (submucosa), the renaming of T2a (muscularis propria) as T2 and T2b (subserosa) as T3, and the subdivision of T4 (serosa) into T4a (penetrates serosa) and T4b (invades adjacent structures). Consequently, the categorization of the T (depth of invasion) is now uniform throughout the gastrointestinal tract, whereas differences remain for the categorization of the N (presence or absence of regional lymph node metastases). The N categories for GC are N0 (no regional lymph node metastasis), N1 (1 to 2 lymph node metastases), N2 (3 to 6 lymph node metastases), N3a (7 to 15 lymph node metastases), and N3b (metastases in 16 or more regional lymph nodes) [19, 20].
Table 6.2
TNM classification of gastric carcinoma
T – Primary tumor |
TX Primary tumor cannot be assessed |
T0 No evidence of primary tumor |
Tis Carcinoma in situ: intraepithelial tumor without invasion of the lamina propria, high-grade dysplasia |
T1 Tumor invades lamina propria, muscularis mucosae, or submucosa |
T1a Tumor invades lamina propria or muscularis mucosae |
T1b Tumor invades submucosa |
T2 Tumor invades muscularis propria |
T3 Tumor invades subserosa |
T4 Tumor perforates serosa or invades adjacent structures |
T4a Tumor perforates serosa |
T4b Tumor invades adjacent structures |
N – Regional lymph nodes |
NX Regional lymph nodes cannot be assessed |
N0 No regional lymph node metastasis |
N1 Metastasis in 1–2 regional lymph nodes |
N2 Metastasis in 3–6 regional lymph nodes |
N3 Metastasis in 7 or more regional lymph nodes |
N3a Metastasis in 7–15 regional lymph nodes |
N3b Metastasis in 16 or more regional lymph nodes |
M – Distant metastasis |
M0 No distant metastasis |
M1 Distant metastasis |
Spreading and Prognosis
Gastric carcinomas can spread by (i) direct extension to adjacent organs, (ii) lymphatic invasion, (iii) blood vessel invasion, and/or (iv) peritoneal dissemination. Intestinal type GCs preferentially metastasize hematogenously to the liver, whereas diffuse type GCs preferentially metastasize to peritoneal surfaces [1]. GCs with mixed histological phenotype exhibit the metastatic patterns of both types.
A recent meta-analysis comparing survival rates after gastrectomy between GC patients from the West and the East from patients recruited into large randomized controlled clinical trials showed an association between type of surgical resection performed in the East and improved survival [30]. The known difference in surgical techniques between the East and the West is one potential variable that may be responsible for discrepancy in outcomes. Noguchi et al. [31] reported a survival difference between high-volume centers in the USA and Japan which was no longer apparent after adjusting for tumor location. Verdecchia et al. [32] demonstrated that the survival of Italian GC patients was inferior to that of Japanese GC patients and that this survival difference disappeared after adjusting for stage. Bollschweiler et al. [33] compared the survival of Japanese and German GC patients and concluded that the country itself was a prognostic factor. Higher frequency of early stage GC and more accurate staging have also been associated with improved survival in Japan compared with Western nations [34].
Early and advanced GCs differ in prognosis. Japanese patients with EGC have an excellent prognosis with a 5-year survival rate exceeding 90 % after surgical treatment. Nevertheless, approximately 2 % of EGC recur after curative resection and lymph node metastases occur in 2–3 % of intramucosal carcinomas [35, 36] and 20–30 % of submucosal carcinomas [37]. Risk factors for lymph node metastasis in EGC include age at time of diagnosis (the younger, the more frequent the lymph node metastases), size greater 20 mm, depressed macroscopic type, grade of differentiation, presence of an ulcer or scar, lymphatic channel invasion, and submucosal invasion by more than 500 μm [35, 37].
Five-year survival rate of advanced GC, the most frequent type in the West, is around 23 % when treated by surgery alone and around 36 % when treatment includes perioperative chemotherapy [38]. For advanced GC, depth of infiltration into the wall (T category of the TNM classification), number of lymph node metastases (N category of the TNM classification), and presence of distant metastases (M category of the TNM classification) remain the strongest prognostic indicators [19, 20]. Lymphatic and venous invasion are also predictors of poor survival in GC. Perineural invasion correlates with T stage and tumor size and may serve as a marker of advanced disease [39].
Genetic Predisposition and Hereditary Syndromes
First-degree relatives of patients with GC are almost three times more likely to develop GC themselves compared to the general population which has been partially attributed to H. pylori infection and to the potential role of IL–1 gene polymorphisms.
Genome-wide association studies have implicated the prostate stem cell antigen (PSCA) gene and the mucin 1 (MUC1) gene as GC susceptibility factors. Approximately 95 % of the Japanese population have at least one of the two risk genotypes, and approximately 56 % of the population have both risk genotypes [40]. Hereditary GC accounts for 1–3 % of GC, and two hereditary syndromes have been described – hereditary diffuse gastric cancer (HDGC) and gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS).
Hereditary Diffuse Gastric Cancer (HDGC)
On the basis of clinical criteria, the International Gastric Cancer Linkage Consortium defined families with HDGC syndrome as families meeting one of two criteria: (i) two or more documented cases of diffuse type GC in first- or second-degree relatives with at least one of them diagnosed before the age of 50 years or (ii) three or more documented cases of diffuse GC in first- or second-degree relatives independent of the age at diagnosis [41]. Women in these families have an elevated risk of lobular breast cancer. The criteria for genetic testing were updated in 2010 [42]. In several HDGC families, a higher incidence of orofacial clefts has been noted [43, 44].
Alterations of the CDH1 gene, which encodes E-cadherin, constitute the genetic causal event in HDGC patients [45]. In clinically defined HDGC patients, CDH1 germline mutations are detected in 30–40 % of cases [42]. Seventy-five to eighty percent of CDH1 mutations are truncating mutations, and the remaining are missense mutations. In addition, large germline deletions have also been found in HDGC families which tested negative for point mutations [46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree