22 Partial Breast Irradiation
No topic is more hotly debated and no treatment approach is more patient-driven in breast cancer therapy than partial breast irradiation (PBI). The following discussion focuses on treatment techniques delivered postoperatively, allowing for confirmation of pathologic staging and margin status. Intraoperative PBI techniques are therefore not discussed here.
Challenging Whole Organ Therapy
The oncologic hypothesis questioning the value of whole organ (i.e., whole breast) irradiation is a timely one in the sequential evolution of local breast cancer management. Localized treatment approaches have previously addressed the entire organ, either through surgery alone (mastectomy) or a combination of surgery and radiation therapy (lumpectomy and whole breast radiation therapy). Surgery for early-stage disease has progressed over the last 30 years from surgical removal of the entire breast with underlying musculature (radical mastectomy), to mastectomy techniques that maintain integrity of the pectoralis muscles (modified radical mastectomy), to breast preservation consisting of removal of the tumor alone with varying but limited amounts of contiguous adjacent breast tissue (quadrantectomy, tylectomy, and lumpectomy). Breast-conserving surgery for breast cancer, which by definition does not resect the entire breast, is always followed by WBI. Trials comparing WBI versus observation after breast-conserving surgery consistently demonstrate that radiation therapy significantly reduces ipsilateral breast tumor recurrence rates compared with breast-conserving surgery alone.1–4
For the majority of solid organ tumors, whole organ treatment renders no additional curative benefit over localized therapy. This is primarily due to the relatively recent appreciation that solid organ malignancies are initiated through a localized malignant transformation on a cellular level, and spread of these clonogens occurs either by local cell division and direct contiguous invasion of adjacent tissue or via lymphatic and hematogenous routes. The latter modalities of spread are best addressed with either systemic therapy or local therapies directed at adjacent lymphatic drainage stations. Lung, esophagus, pancreas, kidney, rectum, and colon cancers are merely some of the common malignancies for which these oncologic principles apply; curative local therapies (i.e., surgery and/or radiation therapy) focus on the location of the tumor itself with a limited amount of surrounding tissue and not on the entire organ. Exceptions to this rule are primarily organs so small that an attempt to achieve both tumor excision and organ preservation is technically impossible, or that treatment of the tumor with margin essentially results in whole organ therapy by default. Cancers of the prostate, cervix, and tonsil are examples of the latter category.
The localized approach to treating the index lesion or gross disease, considered standard in most other solid malignancies, is only now being evaluated for cancer of the breast. Though ripe for investigation, this approach in early breast cancer is associated with several issues unique to this disease and to the patient population. Early-stage breast cancer is curable in most women, and death rates from breast cancer have significantly decreased compared with prior decades.5 Second breast cancers in the decades following initial treatment are a recognized and significant health risk, realized increasingly because of successful control of local and distant disease after treatment of the first breast cancer. Reported rates of such tumors range consistently from 0.5% to 1% per year,6 a seemingly tiny rate in any given year. However, with treatments resulting in increasing cure rates and with overall increasing life expectancy of the population, the probability of such events reaches a meaningful magnitude. For the average woman diagnosed at the age of 60 and expected to live to 80, risk of a second breast cancer is 10% to 20% (without adjuvant/preventive therapies). This cumulative risk only increases for a given patient as the age of diagnosis decreases and life expectancy increases. This is not the case for most other solid organ tumors, in which the lethality rate due to the initial diagnosis is very high or the likelihood of additional remote tumors in the same organ is unusual.
Second cancers are defined both as any tumor in the contralateral breast or as noncontiguous tumors in remote portions of the same breast; however, only the latter is relevant to consideration of the appropriateness of partial breast treatment. Put another way, the key question can be phrased as: Is the “normal” tissue in the ipsilateral breast any different from tissue in the contralateral breast? If not, then from a medical standpoint both should be managed similarly. All molecular and genetic evidence suggests that the malignant potential of uninvolved ipsilateral tissue is the same as that of contralateral breast tissue, and this is supported by the clinical observation that second malignancy rates in the ipsilateral breast are similar to contralateral breast cancer diagnosis rates after WBI.7 Further supporting the idea that “elsewhere” failures in the breast are new primary tumors that develop over time and do not benefit from treatment with WBI is the observation that the incidence of elsewhere failure in the ipsilateral breast is minimally affected by whether WBI is delivered after lumpectomy.8,9 These randomized trials from Canada and Italy document remarkably similar patterns of failure: 0.6% to 0.9% elsewhere failures after WBI and 2.8% to 3.5% elsewhere failures without WBI. Just as there is no indication for treatment of the contralateral normal breast in a woman with early-stage breast cancer, the hypothesis behind PBI states that tissue beyond the index tumor within the breast is no less normal and therefore ought not to require treatment. Randomized trials comparing WBI with PBI are attempting to verify this concept clinically.
The Case for WBI
This perspective is counterbalanced by pathologic data (though predating modern screening and treatment techniques) demonstrating that breast cancer cells can be detected in regions of the breast distant from the index tumor.10,11 With these data in mind, the role of WBI is not only to treat the residual tumor cells immediately surrounding the lumpectomy bed, but also to eradicate microscopic clonogens in remote portions of the breast or in tissue more than 1 to 2 cm from the cavity. Further challenging the field of PBI is the track record of WBI in which excellent local control rates are achieved with minimal acute or long-term morbidity and excellent long-term cosmesis. Therefore, the question arises: What is being accomplished with PBI? Are there any toxicity, cosmesis, or tumor control benefits? If not, then this treatment approach will be beneficial only in that it will be timesaving for the treated patient. The bar for PBI to overcome is therefore set quite high.
Several trials have recently been presented or updated using accelerated WBI fractionation schemas.12–14 These approaches use 13 to 16 fractions delivered over 3 to 5 weeks. The equivalent and excellent tumor control outcomes with these accelerated approaches, accomplished without cosmesis deficits, further chip away at the potential benefit that PBI offers for a patient. Breast-conserving therapy for a woman with early-stage breast cancer no longer requires 5 to 6 weeks of daily radiation therapy. Treatment with WBI can be completed with 13 to 16 treatments delivered over 3 weeks, thus lessening the relative advantage of a 1-week PBI treatment option.
Patient Selection
The clustering of enrolled patients around the most favorable eligibility criteria indicates a hesitancy of treating physicians to enroll younger patients with larger tumors in PBI studies. This was most dramatic in the enrollment of patients in NSABP B39/RTOG 0413 (National Surgical Adjuvant Breast and Bowel Project B 39/Radiation Therapy Oncology Group 0413), the randomized trial comparing PBI with WBI in North America.15 An interim analysis of the patients rapidly enrolled in the first 2 years demonstrated that these included a far greater proportion of node-negative, postmenopausal, and estrogen receptor (ER)-positive patients than anticipated. The protocol was therefore amended to exclude further enrollment of postmenopausal node-negative and ER-positive patients or ER-positive ductal carcinoma in situ patients as of January 2007.
Table 22-1 summarizes the eligibility criteria for selected larger PBI series.16–24 Most publications describe eligibility criteria for PBI as tumors of 3 cm or less, with invasive or in situ histologies and negative surgical margins (no tumor on ink) and negative axillary lymph nodes, as documented by either sentinel lymph node biopsy or axillary dissection. The RTOG studies have included the widest range of eligibility: tumors up to 3 cm, invasive or noninvasive disease, ductal or lobular invasive histologies, and node-positive tumors (up to three positive lymph nodes). NSABP B39/RTOG 0413, which is currently accruing patients, will help identify the appropriateness of PBI for patients across the broader spectrum of breast-conserving therapy, addressing T2, node-positive, ER-negative disease, including presentations in premenopausal women.
There are several consensus documents defining patient selection parameters for PBI25–29 (Table 22-2). They are based on the limited available published experience that addresses primarily postmenopausal women with T1N0 disease—patients with an extremely low risk for systemic and local relapse. Patients with higher rates of local and distant relapse are excluded, despite the fact that no data suggest that these patients have higher rates of ipsilateral breast tumor recurrence with PBI than lower-risk patients treated with PBI. In other words, patients not described in these definitions are not ineligible, but have not been evaluated yet in the clinical setting. The best that can be done at this point is to summarize the available clinical experience and encourage trial design and enrollment that will scientifically address these issues in the future.
The issues related to limiting tumor size for PBI consideration relates not only to a concern for increased breast cancer recurrence, but to technical factors as well. As the resection volume increases, the surface of the resection cavity and the amount of tissue requiring irradiation with PBI significantly increase as well. Researchers have made an effort to limit the proportion of treated normal breast tissue to ensure that the treatment is truly “partial” breast irradiation. For node-positive patients, an added consideration is whether or not regional nodal irradiation is an important component of therapy. For patients with node-positive disease who have undergone standard axillary dissection, regional nodal relapse is extremely rare.30 Treatment with PBI without regional nodal irradiation is therefore a reasonable consideration and is being investigated.
Impact of Technologic Advances and Techniques
WBI was developed primarily as a replacement for whole organ surgical therapy—to allow for breast conservation. It also matured during an era when imaging and treatment planning technologies were comparatively crude. The trials of WBI were conducted in the 1970s and early 1980s when two-dimensional treatment planning was the norm; computed tomography (CT) was in its infancy as a diagnostic tool and not yet integrated into radiation therapy treatment planning systems. When these imaging and treatment planning technologies matured and were ultimately paired together, conformal or three-dimensional (3D) radiation therapy became possible. The ability to identify a target on multiple CT images, visualize it in 3D space, and relate the target to treatment parameters and dosimetry set the stage for feasibility of a PBI approach. Stated another way: the ability to deliver PBI with any reproducible accuracy wasn’t possible until the last decade, when imaging and treatment planning software became adequately sophisticated. All modern marketed software programs allow for 3D treatment plan design, whether with external-beam radiation therapy (EBRT) or brachytherapy. Although PBI has been attempted in past decades, it is no coincidence that the explosion of interest and delivery of PBI has occurred during this unique era in technologic development of the radiation therapy field.
PBI can now be delivered in a multitude of ways (Table 22-3), and can be best categorized into three distinct techniques: interstitial brachytherapy, intracavitary brachytherapy, and EBRT. Multicatheter interstitial brachytherapy is the technique with the longest track record in the modern era. Intracavitary brachytherapy (primarily with the MammoSite device) was the next to come into clinical practice. The newest approach with the shortest follow-up and experience is EBRT. All these approaches deliver radiation therapy to the 1 to 2 cm of tissue surrounding the lumpectomy cavity.
Table 22-3 Partial Breast Irradiation Techniques
Interstitial Multicatheter Brachytherapy
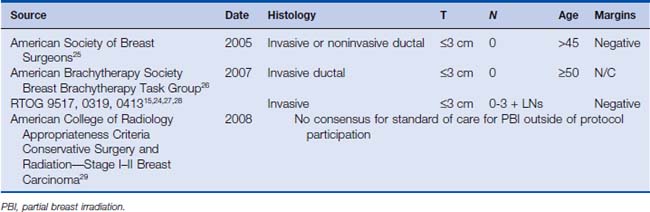
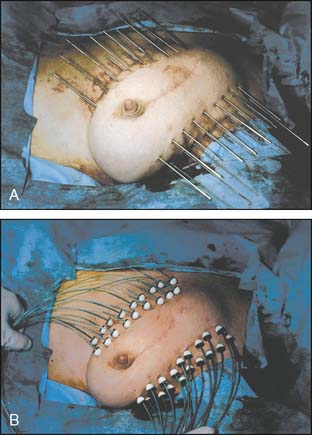
Interstitial multicatheter brachytherapy was pioneered and promoted in the United States by Dr. Robert Kuske from the Ochsner Clinic in the early 1990s. His initial experience31 led to numerous other single institution experiences, and ultimately to a phase II cooperative group study investigating this approach (RTOG 9517).32 These implants were typically designed in two planes situated on the superficial and deep aspects of the lumpectomy cavity (Fig. 22-1). Templates were subsequently developed to allow for more even distribution of catheters, coverage of the target volume, and ease in catheter placement. This approach generally requires 15 to 20 catheters that traverse through the breast, resulting in excellent conformal coverage of the target volume (Fig. 22-2). The drawbacks of multicatheter brachytherapy are that the radiation oncologist must be comfortable with both invasive procedures and sophisticated brachytherapy treatment planning with appropriate physics support. These requirements are generally referenced as limiting this technique’s popularity. Supporting this limitation is the observation that only 10 centers enrolled patients in RTOG 9517.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree