11. Parenteral nutrition
Paula Murphy
LEARNING OBJECTIVES
By the end of the chapter the reader will be able to:
• Appreciate the indications for parenteral nutrition;
• Gain an insight into the parenteral nutrition solutions available and the evolution of some special substrates;
• Understand the different routes by which parenteral nutrition can be delivered and the venous access devices available;
• Understand the complications associated with parenteral nutrition delivery and how they may be avoided;
• Gain a practical understanding of how a patient receiving parenteral nutrition should be monitored; and
• Appreciate the indications, prevalence and complications associated with home parenteral nutrition.
Introduction
Parenteral nutrition (PN) refers to the provision of nutrients by the intravenous route and was introduced by Dudrick in 1967. 1 Since then it has developed as an effective feeding technique and has been widely implemented for feeding patients with both functioning and non-functioning gastrointestinal tracts. Its use in patients with functioning gastrointestinal tracts occurred for a number of reasons including its relative ease of administration, poor understanding of gut function and enteral feeding techniques, and a limited range of enteral feeding devices. PN use is now declining with recognition of its inherent risks and evidence for the superiority of enteral nutrition (EN). Nonetheless PN is a life-saving therapy for many patients with gastrointestinal (GI) failure.
The choice of EN versus PN is no longer an issue. There are no data from randomised trials to support the use of PN in patients with an intact gastrointestinal tract, 2 and PN may be associated with increased complications when given to patients not malnourished. 3 Worthy of consideration, however, is that many of the trials comparing enteral with parenteral nutrition are not comparable in terms of energy intake. Studies reported that an increased incidence of sepsis in patients receiving PN were fed more energy compared to enterally fed patients. 4.5. and 6. Overfeeding easily occurs with PN and increases the risk of complications. Excess delivery of nutrients by the enteral route as well as the parenteral route is associated with complications and should be avoided. Notwithstanding this, where artificial nutrition support is required, it is established that enteral tube feeding is the safer, cheaper, less demanding and a more ‘physiological’ option. 7 Although the exact reasons for the effectiveness of enteral over parenteral nutrition in patients with functional gastrointestinal tracts are not yet fully established, results from animal and human studies clearly show parenteral formulas to be less supportive of the immune and gastrointestinal systems.
Indications for parenteral nutrition
PN is an essential adjunct to the management of patients who are unable to obtain adequate nutrition via the enteral route. It should be reserved for patients who need support but have either a non-functioning or a non-accessible gastrointestinal tract. 7 This might include patients with short bowel syndrome, intestinal ischaemia or infarction, intestinal obstruction, severe diarrhoea, motility disorders (e.g. scleroderma), acute intestinal failure, large volume fistula output and inability to access the gastrointestinal tract. It has been suggested that up to 70% of patients receiving PN may have impaired gastric emptying and reduced colonic motility but adequate small bowel function and thus could be fed by the enteral route. 2 There is a subset of patients where the GI tract is accessible but an adequate nutrient intake cannot be achieved; in these cases supplementary PN can be provided. Optimal timing and composition of PN in these cases requires further study. If individuals are well nourished there is no evidence that withholding PN for seven days is harmful; providing PN during this period would only result in an increased risk of infection.
Administration of parenteral nutrition
Factors to be considered when planning for a patient to receive PN are outlined in Box 11.1.
 Box 11.1
Box 11.1 1. Nutritional requirements
2. Baseline metabolic parameters
3. Anticipated duration of PN
4. Accessibility of veins
5. Venous access device
6. Complications of therapy (risks should be weighted against the benefits)
Nutrient solutions should be administered through a dedicated feeding line using a volumetric pump with occlusion and air-in-line alarms. 7 Administration sets should be changed every 24 hours. 8 PN can be administered continuously (over 24 hours) or cyclically (over 10–18 hours). For patients receiving peripheral PN via peripheral venous cannulae, cyclic delivery of PN should be considered with planned routine cannula change (every 1–2 days) to reduce the risk of peripheral vein thrombosis (PVT). Cyclic PN has the advantage of facilitating patient mobility, which is particularly important for the quality of life of patients receiving home PN. It has also been suggested that cyclic PN may be helpful in the management of PN-associated liver disease. 9 For patients receiving PN centrally, there is evidence that continuous delivery may lead to better nutrient balance. 10 It should be noted that this study included major surgery patients and may not be applicable to patients requiring long-term PN where metabolic conditions are different.
Parenteral nutrition solutions
Total parenteral nutrition (TPN) implies that all macronutrient (carbohydrate, amino acid and lipid) and micronutrient (electrolytes, vitamins and trace elements) requirements are met by a solution administered into a peripheral or central vein. TPN admixtures are referred to as all-in-one (AIO) solutions containing all the required nutritional components. Standardised fixed feeding regimens or individually compounded admixtures are available. These are described in more detail elsewhere. 11 Vitamins, trace elements and electrolytes may be added to both regimens but must be done under controlled pharmaceutical conditions and not at ward level. 7 Individually compounded admixtures are manufactured under strictly controlled aseptic conditions in a suitable pharmacy manufacturing unit. There is no evidence that outcome is improved with either regimen. It is recommended that patients’ nutritional requirements be established by suitably trained personnel before the PN regimen type is decided. 7,12 Patients’ requirements should be balanced with standardised PN formulations to enhance patient safety and reduce both ordering and compounding errors. Ultimately a safe PN system must be in place to minimise procedural incidents and maximise the ability to meet individual requirements.
Macronutrients in parenteral nutrition
Carbohydrate
Carbohydrate in PN is provided by glucose, a cheap energy source available in a range of concentrations, 5–70%. While the inclusion of some carbohydrate is essential for Central Nervous System (CNS) function, the maximum glucose oxidation rate, 4–5 mg/kg /min/day, should not be exceeded as this may result in hyperglycaemia, hepatic steatosis and impaired respiratory function with increased CO 2 production. Glucose tolerance is impaired in patients with sepsis and concurrent insulin treatment may be necessary to prevent hyperglycaemia.
Protein
The nitrogen component of PN is supplied as a mixture of l-amino acids with essential amino acids supplying approximately 40% of the total amino acid nitrogen. Solutions enriched with certain amino acids have become available, e.g. glutamine. Glutamine, although not an essential amino acid, may become essential during metabolic stress. There are problems providing glutamine in PN solutions due to its instability and low solubility in aqueous solutions. At present there is no evidence that supplementation is harmful but further study is required to provide evidence of benefit. Large multicentre trials are ongoing and results expected within the next few years, e.g. Scottish Intensive Care Glutamine or SeleNium Evaluative Trial (SIGNET) and the Scandinavian Critical Care Trials group study. The former is a randomised trial of glutamine-supplemented PN for critically ill patients and is being carried out throughout Scotland involving intensive care and high dependency units. The latter is a large randomised double-blind placebo controlled study of IV glutamine supplementation in intensive care patients in Scandinavia.
Lipid
Lipid in PN solutions provides non-glucose energy, minimises respiratory and metabolic stress, prevents essential fatty acid deficiency and allows peripheral infusion of nutrients. Lipid can accumulate in the reticuloendothelial system, impairing its ability to remove bacteria and endotoxins and increasing susceptibility to infection; consequently it is recommended that lipid content of PN should not exceed 1.5 g/kg/day. 13 Soybean emulsions have been used as the lipid source for more than 30 years. They contain a high proportion (> 60%) of polyunsaturated fatty acids (PUFA), linoleic acid (52–54%) and alpha linolenic acids (7–9%). It has been suggested that soybean oil-based emulsions represent an imbalanced fatty acid supply with an excess of n6 fatty acids, which under conditions of stress may be proinflammatory, and promote platelet aggregation and vasoconstriction. 14 The ideal lipid emulsion would supply essential fatty acids, provide easily metabolisable energy and have anti-inflammatory properties. Novel lipids promising to modulate inflammatory responses and improve outcomes have been developed. These include emulsions containing MCT, olive oil and fish oil in various combinations as a partial replacement for soybean oil. Supplying n-3 fatty acids may have the opposite effect to fatty acids of the n6 series. Eicosanoids derived from the former tend to promote vasodilation, inhibit platelet aggregation and reduce inflammation. Consequently fat emulsions enriched with n-3 fatty acids would be expected to have a favourable impact on outcome in critically ill patients receiving PN. 15 n-3 PUFA-containing triglycerides are poorly hydrolysed by lipoprotein lipase; consequently pure fish oil-containing emulsions must be infused at a very low rate to avoid triglyceride accumulation in the circulation. MCT are excellent substrates for lipoprotein lipase-mediated hydrolysis, facilitating plasma triglyceride clearance. 16 They are not suitable as the sole lipid source as they do not include essential fatty acids and have a tendency to cause metabolic acidosis. These problems have been overcome by combining MCT and fish oil in emulsions. Lipid preparations based on olive oil can also be used to decrease the intake of PUFA. Such novel lipids have been shown to be safe and may offer some advantages over the use of soybean oil alone but there is a lack of sufficient data on immunologic and clinical endpoints. 17 More work is needed to evaluate these emulsions before recommendations can be made.
Micronutrients in parenteral nutrition
The intravenous (IV) administration of trace elements poses a risk of toxic effects as the regulatory absorptive mechanism of the intestine is bypassed. An adequate supply of micronutrients is essential for patients on PN to prevent clinical and subclinical deficiency states. Commercially prepared mixtures that provide well-balanced amounts of all essential vitamins and trace elements are available. These commercial preparations are based on guidelines for essential trace element preparations for parenteral use developed by the Nutrition Advisory Group of the Department of Foods and Nutrition, American Medical Association in 1979. 18 Requirements for parenteral trace elements will vary among patients depending on clinical and metabolic status and the need to replace any losses from the GI tract. Further supplements may be appropriate in certain circumstances. For example, starved patients may require additional thiamine as reserves would be expected to be low, pancreatic fistula fluids have a high content of micronutrients especially zinc, biliary fistula fluid is rich in copper and manganese and thus these micronutrients are lost with fluid losses. Knowledge of the stability of micronutrients when mixed with other components of the PN solution and of the effects of the type of container and conditions of storage is essential to ensure that patients actually receive the micronutrients they require. For example, certain vitamins within PN solutions may undergo degradation by sunlight, e.g. vitamin A and E. Solutions should be protected from light by a light-shielding cover. Further losses of vitamin A may be caused by adsorption of vitamin A to the plastic container. 19 Ascorbic acid is the least stable vitamin in solution reacting with oxygen to form dehydroascorbic acid, a reaction catalysed by copper and iron. The benefit of individual micronutrient provision in larger amounts, in particular those known to affect free radical scavenging mechanisms or immune function, continues to be a matter of debate. Controlled trials are required before recommendations can be made.
Parenteral nutrition access routes
Insertion of a catheter for PN should never be an emergency procedure. Patient consent should be obtained and the risks and benefits explained in advance. Administration may be via peripheral or central routes.
Peripheral parenteral nutrition (PPN)
Peripheral parenteral nutrition refers to the administration of nutrients via superficial veins. Use of these veins reduces the risks associated with central line placement.
The principal factors influencing the selection of this route of venous access are: the patient’s nutritional requirements; accessibility of veins and anticipated duration of PN. PPN will not be suitable for patients with high nutrient requirements, those requiring low volume solutions or those where the anticipated duration of PN is likely to be > 14 days. It should be considered when the duration of PN is anticipated to be short and the patient does not need central venous access for other reasons.
Choice of vein
The cephalic, basilic or median cubital veins of the forearm are the veins of choice for the peripheral delivery of nutrients (Figure 11.1). Peripheral veins of the lower extremities should be avoided due to higher risks of thrombophlebitis and because of the need to confine the patient to bed. 20 The non-dominant forearm should be used if possible. Frequently in practice, this route of access may not be suitable in very sick patients due to the presence of inflammation from previous cannulation, thrombosis or oedematous limbs making cannulation difficult.
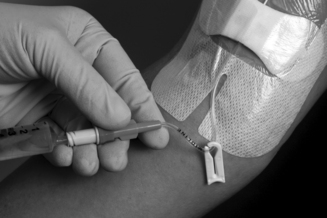 |
| Figure 11.1 • (with kind permission from Vygon Inc). |
Choice of device
Venous access may be obtained through a Teflon or PVC cannula (18–20 G) or an ultrafine polyurethane catheter (19–23 G). If the former is used, a short extension set should be attached to facilitate feeding. It is recommended that cannulae be covered with a sterile adhesive dressing and re-sited every 1–2 days to delay the onset of PVT. 7 This is time consuming for staff and uncomfortable for the patient. In some centres it is common practice to leave the cannula in place until the first sign of thrombophlebitis. An alternative is to use an ultrafine (19–23 G) silicone or polyurethane catheter, 10–15 cm in length that is inserted into a vein in the antecubital fossa. These are often referred to as mid-lines. Placement should only be by a competent practitioner. They have the advantage of being less thrombogenic, longer lasting and more comfortable for patients. There are a number of devices available with different dwell times recommended by the manufacturers. Blood aspirate is necessary to confirm placement. Although they are more expensive initially than cannulae, they may be longer lasting and less labour intensive for staff. Peripheral devices can be placed on the ward with a strict aseptic technique and appropriate skin preparation. Strict hand washing and an aseptic technique are important for all subsequent manipulations of the device.
Choice of nutrient solution for peripheral administration
Hyperosmotic solutions are poorly tolerated by peripheral veins, causing pain, thrombophlebitis and thrombosis. In most nutrient solutions the osmotic components are glucose, amino acids and electrolytes. The inclusion of lipid and an increase in volume of solution will reduce osmolarity. In addition, lipid emulsion-based admixtures may also have a pH better tolerated by small vessels. 7 Consideration should be given to electrolyte content, as additions of electrolytes will increase the tonicity and affect the pH. Solutions < 1200 mosm/L have been shown to be tolerated. 21 A typical PN regimen for peripheral infusion is outlined in Box 11.2.
 Box 11.2
Box 11.2 • 2500 mL in a 3 compartment bag (AIO) comprising:
○ 1000 mL of 5.5% amino acid solution
○ 1000 mL of 20% glucose solution
○ 500 mL of 10% lipid emulsion
The contents of the three compartments are mixed and the tertiary mixture provides:
• 9.1 g N 2
• 1520 total calories (800 glucose calories, 500 lipid calories)
• 53 mmol sodium
• 40 mmol potassium
• 5.5 mmol magnesium
• 5.0 mmol calcium
• 17.5 mmol phosphate
Osmolarity is 750 mOsm/L (this will alter with further electrolyte additions).
pH is 6.
Source: OliClinomel N4-550E 2500ml, Baxter Healthcare, UK. Reproduced with kind permission.
Care of peripheral devices
Irrespective of whether a cannula or a catheter is used, the site should be monitored daily for signs of infection or thrombophlebitis and removed if there are any early signs of inflammation. Due to the small internal diameter of devices they should be flushed on discontinuation of the infusion as the risk of occlusion is high. Staff training on peripheral cannula management is important to reduce morbidity and loss of peripheral veins.
Complications of PPN
Infectious complications
The incidence of sepsis in association with PPN can be reduced by strict aseptic technique and adherence to protocols for catheter insertion and care.
Peripheral vein thrombophlebitis
The most common complication associated with PPN is PVT (inflammation of a vein just under the skin). It is characterised by redness, pain, swelling and tenderness along a part of the vein. A myriad of factors outlined below are associated with its development. Arguably, the most important factor is mechanical trauma causing endothelial damage within the vein. Trauma may occur as a consequence of the venepuncture, as a result of the presence of the cannula within the vein or because of the irritant effects of the infusate on the vein wall. The resulting trauma may cause the release of inflammatory mediators, activation of the clotting cascade and subsequent phlebitis and thrombosis.
Factors associated with PVT
Feed composition
The incidence of thrombophlebitis is related to the osmotic content of the infused solution as well as to the osmolarity rate (product of osmolality and infusion rate). 22 The availability of lipid-based formulations enable feeds containing relatively high concentrations of solute to be administered safely.
Choice of cannula
Short Teflon cannulae such as those used to administer intravenous crystalloid are associated with phlebitis of up to 100%. 21 Use of ultrafine (22 or 23 G) polyurethane cannulae have been associated with a low incidence of PVT, approximately 15%. 23,24 The latter are narrow and flexible compared with standard Teflon cannulae and may cause less mechanical trauma to the vein. The tip of the fine bore catheter lying in a large diameter vessel may result in a greater dilution of the nutrient solution.
Timing of infusion
Over time PVT will develop in any vein in which there is an indwelling cannula. 25 It is not surprising therefore that cyclical feeding (usually a 12-hour infusion and 12-hour break) with an elective change of cannula has been found to be associated with reduced incidence of thrombophlebitis (0–18%). 26.27. and 28. These studies were small, however, and have been considered to present ‘limited scientific evidence’. 29 Nonetheless, the conclusion reached by NICE was that cyclic delivery of PN should be considered when using peripheral venous cannulae with planned routine catheter change (every 1–2 days). 7 Cyclic feeding is time consuming for staff, however, and may be painful and uncomfortable for the patient.
Pharmaceuticals
Heparin and hydrocortisone added to the PN bag may have a significant and synergistic effect in reducing thrombophlebitis. 30,31 The addition of heparin to some lipid-based feeds results in the aggregation of lipid particles. Consequently it cannot be routinely recommended until stability studies have been undertaken with the prescribed formulation. 32 Glyceryl trinitrate patches or gels containing non-steroidal anti-inflammatory drugs placed over the vein distal to the insertion site have been shown to reduce thrombophlebitis in patients receiving crystalloid infusions. 33,34 This acts by venodilation and consequently increases blood flow. These drugs increase the cost of PN and necessitate additional pharmacy and nursing interventions. Although some evidence exists to support their use, it is insufficient to warrant routine use at present.
Central parenteral nutrition
Central parenteral nutrition refers to the administration of nutrients via central veins. Because of the risks associated with central vein cannulation, it should be considered only when peripheral access is not feasible or not appropriate (see section on complications of PN overleaf). 7 Central vein cannulation should be by well-trained personnel utilising ultrasound guidance and using aseptic technique under strict aseptic conditions.
Central PN is likely to be used in the following patient categories.
1. Patients with special nutrient requirements, e.g. fat free, high nutrient requirements or reduced fluid requirements;
2. Patients in whom the expected duration of PN is > 14 days;
3. Patients who do not have suitable peripheral veins; and
4. Patients who already have a central venous access device in place with a lumen that can be dedicated to feeding.
Choice of vein
Access to the superior vena cava can be gained through the internal jugular vein, the subclavian vein or through the peripheral veins in the arm (see Periphally Inserted Central Catheters below). The subclavian vein is the most commonly used access route for long-term PN. Cannulation of this vein creates higher risk of pneumothorax, requiring it to be used by experienced personnel. The tip of the catheter should be placed in the distal upper vena cava just above the right atrium. The internal and external jugular veins may also be used but the position of the catheter can be uncomfortable for the patient. Nonetheless both are clearly visible veins, easy to locate and consequently frequently used for emergency access. Another option is the femoral vein. This is used only in the absence of other suitable veins due to the high risk of infection and catheter-related venous thrombosis.
Choice of central venous access device
A central venous access device (CVAD) is defined as having its tip located in the superior vena cava.
Ideally a single lumen dedicated CVAD should be used for PN administration. In practice most acutely unwell patients requiring PN may have a previously inserted CVAD in place, placed for example during surgery, or will require other intravenous access. In this case a multilumen CVAD will be required with a lumen dedicated to PN. Device selection is based on patient factors and the anticipated duration of therapy. The ideal catheter material for long-term central venous access is chemically inert, non-thrombogenic, flexible and radio-opaque. 35 Most commonly used CVADs are silicone, polyurethane or PVC and have a diameter between 18 and 22 G. They may have single, double or triple lumens. Some devices are coated by antibiotics, which are slowly released in order to decrease the risk of infection associated with migration of bacteria over the outer surface of the catheter. CVADs are also available impregnated with chlorhexidine and silver sulfadiazine or other antimicrobial agents, and may be associated with a rate of infection lower than that of untreated devices. These are more expensive and not a substitute for good catheter care.
Central venous access devices: available options
Non-tunnelled devices
Non-tunnelled devices are the most commonly used CVADs. For the purpose of PN delivery they may be used when the duration of PN is likely to be less than 3 weeks. These are often inserted into the internal jugular vein, but can also be inserted into subclavian or femoral veins. This provides the straightest and shortest route of insertion, thereby reducing the risk of malposition. The external jugular can also be used but cannulation can be more difficult. These devices provide reliable access and as they can have up to five lumens, they are ideal for patients requiring multiple therapies (see Figure 11.2). However, they can be uncomfortable, as they are sutured in place, and patient head movements and the weight of administration sets can cause pulling on the sutures. They can also be difficult to dress, which may lead to movement of the catheter and subsequent mechanical thrombophlebitis.
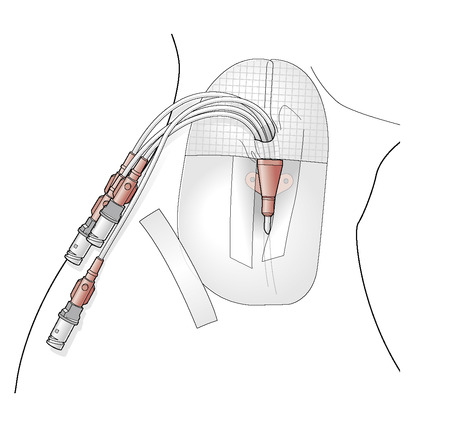 |
| Figure 11.2 • |
Subcutaneously tunnelled devices
These are more commonly used for longer periods of therapy. The subcutaneous tunnelling of CVADs was introduced in the 1980s in an attempt to reduce the risk of catheter-related infection. It was envisaged that infection could be reduced by decreasing bacterial progression on the outer surface of the CVAD between the exit site and the intravascular part of the device. A skin-tunnelled CVAD is usually inserted via the jugular or subclavian vein and advanced into the superior vena cava so that the tip is lying at the junction of the right atrium. The technique used will depend on the type of device used and the manufacturer’s recommendation. There is evidence that subcutaneous tunnelling of CVADs may reduce the risk of catheter colonisation; consequently for long-term PN (> 30 days) subcutaneous tunnelling is recommended. 7,36 Tunnelling is not a substitute for a strict aseptic technique when caring for CVADs. It has the added benefit of ensuring the line is firmly fixed in position and allows easier care of the exit site. Many devices have a Dacron cuff, which over a period of time (approximately 3 weeks) will fibrose with the subcutaneous tissue within the tunnel. The device should be secured with sutures that must remain in place until the Dacron cuff has fibrosed or the device will fall out once the sutures are removed. Most cuffed CVADs require surgical removal by blunt dissection under local anaesthetic. Gentle traction is used by some practitioners if the device has been in place for less than 14 days. Caution must be employed with this approach, as there is a risk of causing trauma to the patient if excessive force is used.
Peripherally inserted central catheters (PICCs)
An alternative means of gaining central venous access is by using a PICC. The PICC is an intermediate to long-term non-tunnelled CVAD with an average life span of 3–6 months. Catheters are silastic or polyurethane and with small diameter, 20–22 G. A PICC can be open-ended with a clamp or may have a Groshong valve at the tip. It is introduced into the basilic or cephalic veins and the tip lies in the superior vena cava. It is essential to remember that although it is inserted peripherally, the tip of a PICC is in the same position as a CVAD inserted infraclavicularly, and must therefore be cared for with the same strict aseptic protocol. The main advantage of a PICC is the avoidance of the risks of direct jugular or subclavian catheterisation and its relative ease of insertion; nurses with good cannulation skills can quickly learn the insertion technique. They can be used for short- or long-term episodes of PN and are particularly useful for patients in whom infraclavicular placement of a CVAD is not feasible, e.g. infection, thrombosis, or patients with respiratory distress. For successful placement, patients need to have good IV access in the ante-cubital fossa. Catheters have a maximum of two lumens and so are unsuitable for patients requiring simultaneous treatments.
Only one randomised controlled trial comparing the efficacy of PICC versus other directly placed CVADs has been published. 37 This study included 102 patients requiring PN and found PICCs to be associated with a greater number of difficult insertion attempts ( p < 0.05), clinically evident thrombophlebitis ( p < 0.01) and malposition on insertion ( p < 0.05). PICC use is nonetheless often successful and a useful alternative to infraclavicular placement.
Totally implanted vascular access devices (TIVADs)
An implanted subcutaneous titanium or plastic port is another option and may be associated with a lower rate of infection (Figure 11.3). 38 These are suitable for patients requiring long-term intermittent venous access and are most commonly inserted in the chest or in the antecubital fossa of the arm. The port is placed by making an incision into the patient’s skin, creating a subcutaneous pocket. The device is then anchored, with sutures, to the underlying muscle and the catheter tunnelled under the skin until it reaches the desired venous access point. The overlying skin is then surgically closed.
 |
| Figure 11.3 • |
Only trained personnel should access the device and a non-coring angled needle should be used to pierce the skin and enter the septum of the port. The main advantage of the system is that it causes very little disturbance to daily activities. The port must be removed under local or general anaesthetic.
Choice of nutrient solution for central vein administration
The delivery of nutrient solutions into a large diameter high flow vein such as the superior or inferior vena cava enables highly concentrated nutrient solutions to be infused.
A typical all-in-one parenteral solution that may be used for patients with higher nutrient requirements and central venous access is outlined in Box 11.3. Ready made all-in-one (AIO) bags require vitamin and minerals to be added in a septic suite in pharmacy.





