Parathyroid carcinoma is a malignant neoplasm affecting 0.5% to 5.0% of all patients with primary hyperparathyroidism. Since it was first described by De Quervain in 1904 to this day, it continues to defy diagnosis and treatment because of its rarity, overlapping features with benign parathyroid disease, and lack of distinct characteristics. En bloc surgical extirpation of the tumor with clear margins remains the best curative treatment. Although prolonged survival is possible with recurrent or metastatic disease, cure is rarely achievable. Efficacy of adjuvant therapies, such as radiotherapy and chemotherapy, in management of persistent, recurrent, or metastatic disease has been disappointing.
Key Points
- •
Parathyroid carcinoma is a rare disease.
- •
Severe hypercalcemia, very high parathyroid hormone, and palpable mass are suspicious for parathyroid carcinoma.
- •
Intraoperative findings may reveal a hard and fixed mass adherent to the thyroid gland and occasionally lymph node suspicious for metastatic tumor.
- •
The pathologic diagnosis may be difficult, as atypical parathyroid adenoma may mimic carcinoma.
- •
The best treatment would be appropriate surgical resection with the ipsilateral thyroid node and excision of the lymph nodes if suspicious.
- •
Adjuvant therapy has very little impact but postoperative radiation therapy may be indicated in locally aggressive tumor or bulky nodal metastases.
Introduction
Parathyroid carcinoma is a rare endocrine neoplasm. Its incidence ranges from 0.5% to 5.0% of the cases with primary hyperparathyroidism. Parathyroid carcinoma commonly has an indolent growth with a tendency for local invasion, and it occurs with equal frequency in men and women. The age at presentation for parathyroid carcinoma compared with adenoma is reported to be a decade earlier. It was first described by the Swiss surgeon Fritz De Quervain. In 1904, he described a case of nonfunctioning metastatic parathyroid carcinoma, and 26 years later, Sainton and Millot described the first case of functioning metastatic parathyroid carcinoma. Armstrong in 1938 described another patient with metastatic parathyroid carcinoma and hypercalcemia, and in 1968, Holmes and colleagues reviewed 50 cases of parathyroid carcinoma reported in the literature. Of those, 46 tumors were functioning with a mean serum calcium level of 15.9 mg/dL. Fewer than 10% of parathyroid carcinoma cases present as nonfunctional tumors. Although most patients with parathyroid carcinoma present with hyperparathyroidism, a diagnosis of hyperparathyroidism attributable to carcinoma may be difficult to arrive at preoperatively or even intraoperatively. In many cases, because of the rarity of the disease and clinical features mimicking benign parathyroid pathology, preoperative diagnosis of parathyroid carcinoma is difficult. In addition, the pathologic diagnosis of malignancy is challenging; however, severe hypercalcemia combined with high parathyroid hormone levels and gross operative findings should arouse suspicion of parathyroid carcinoma. This article reviews the relevant literature on parathyroid carcinoma in an attempt to provide diagnostic and therapeutic strategies.
Introduction
Parathyroid carcinoma is a rare endocrine neoplasm. Its incidence ranges from 0.5% to 5.0% of the cases with primary hyperparathyroidism. Parathyroid carcinoma commonly has an indolent growth with a tendency for local invasion, and it occurs with equal frequency in men and women. The age at presentation for parathyroid carcinoma compared with adenoma is reported to be a decade earlier. It was first described by the Swiss surgeon Fritz De Quervain. In 1904, he described a case of nonfunctioning metastatic parathyroid carcinoma, and 26 years later, Sainton and Millot described the first case of functioning metastatic parathyroid carcinoma. Armstrong in 1938 described another patient with metastatic parathyroid carcinoma and hypercalcemia, and in 1968, Holmes and colleagues reviewed 50 cases of parathyroid carcinoma reported in the literature. Of those, 46 tumors were functioning with a mean serum calcium level of 15.9 mg/dL. Fewer than 10% of parathyroid carcinoma cases present as nonfunctional tumors. Although most patients with parathyroid carcinoma present with hyperparathyroidism, a diagnosis of hyperparathyroidism attributable to carcinoma may be difficult to arrive at preoperatively or even intraoperatively. In many cases, because of the rarity of the disease and clinical features mimicking benign parathyroid pathology, preoperative diagnosis of parathyroid carcinoma is difficult. In addition, the pathologic diagnosis of malignancy is challenging; however, severe hypercalcemia combined with high parathyroid hormone levels and gross operative findings should arouse suspicion of parathyroid carcinoma. This article reviews the relevant literature on parathyroid carcinoma in an attempt to provide diagnostic and therapeutic strategies.
Anatomy, etiology, and epidemiology
The parathyroid glands are endodermal in origin and develop from the dorsal wing of the third and fourth pharyngeal pouches. They produce parathyroid hormone (PTH), which regulates the circulating level of calcium through intestinal and renal absorption and bone remodeling. There are typically 4 parathyroid glands; however, supernumerary glands and fewer than 4 glands have been reported. The superior parathyroid glands originate from the fourth pharyngeal pouch, and they attach to the posterior surface of the inferiorly migrating thyroid. They have a much shorter migration distance compared with the inferior parathyroid glands, accounting for their more predictable location. The dorsal wing of the third pharyngeal pouch gives rise to the inferior parathyroid glands, whereas the ventral wing gives rise to the thymus during the fifth week of gestation. Both primitive glands join the thymus as it travels caudally and medially to its final position in the mediastinum. This migration of the inferior parathyroid glands with the thymus results in the inferior parathyroid gland to be in a plane that is usually ventral to that of the superior parathyroid glands. For this reason, ectopic inferior parathyroid glands can be found anywhere along this large area of descent up to the superior border of the pericardium.
Etiology of parathyroid carcinoma is not known. There is an association between multiple endocrine neoplasia type 1, the autosomal dominant form of familial hyperparathyroidism and an increased risk of parathyroid carcinoma. Additionally, there have been reports of carcinoma occurring in an adenoma, hyperplastic gland, or in patients with history of neck irradiation and end-stage renal disease. In the report by Koea and Shaw, 1.4% of the patients with parathyroid carcinoma had prior history of neck irradiation; however, robust causal relationship between parathyroid carcinoma and radiation or lifestyle factors has not been described.
Parathyroid carcinoma by all means is a rare disease with the reported incidence ranging from 0.5% to 5.0%. There may be a geographic variation in the distribution of this disease, with reported incidence of about 1% in Europe and the United States and about 5% in Japan and Italy. Hundahl and colleagues in their review of the National Cancer Data Base (NCDB) between 1985 and 1995 identified 286 cases of parathyroid cancer, accounting for 0.005% of the total NCDB cancer cases. This variation may represent a discrepancy in histopathological and clinical criteria in recognition of malignancy. Parathyroid carcinoma occurs with equal frequency in men and women, whereas a higher female-to-male ratio (3–4:1) has been reported in parathyroid adenoma. On average, it presents a decade earlier than parathyroid adenoma during the fourth and fifth decade of life; however, these demographic data have no value in diagnosing parathyroid carcinoma. In the NCDB study, the investigators were unable to detect any disproportionate clustering by race, income level, or geographic region in their study population. The median age of this cohort at presentation was 55 years with equal gender distribution.
Molecular Pathogenesis
Although the etiology of these tumors remains unclear, mutations of both oncogenes and tumor suppressor genes have been associated with the development of parathyroid tumors. Some of these genes include the oncogene cyclin Dl, retinoblastoma, and the p53 tumor suppressor gene. Oncogenes in locations 1q, 5q, 9q, 16p, 19p, and Xq and tumor suppressor genes in locations 1p, 3q, 4q, 13q, and 21q may be involved in the pathogenesis of parathyroid carcinoma. Cyclin D1 or PRAD1 ( p a r athyroid ad enoma 1) is an oncogene that is located at chromosome 11q13 and is involved in cell cycle regulation. A chromosomal rearrangement of the cyclin D1 gene with the regulatory region of the PTH gene has been reported in 5% of parathyroid adenomas with the cyclin D1 oncoprotein being overexpressed in 18% to 40% of adenomas. Similarly, overexpression of the cyclin D1 oncogene is observed in up to 91% of the parathyroid carcinoma specimens as reported by Vasef and colleagues and only in 39% and 61% of the adenoma and hyperplasia specimens, respectively. Although this association appears to be strong, direct causality between cyclin D1 and parathyroid carcinoma has not been established. It has been shown that altered expression of the Rb (retinoblastoma) gene is common in parathyroid carcinoma and is likely to be an important contributor to its molecular pathogenesis. In a study of 11 parathyroid carcinoma specimens, all lacked the Rb allele on chromosome 13. In another study, by Pearce and colleagues, allelic deletions of the 13q12–14 region involving both the Rb gene and the hereditary breast cancer susceptibility gene (BRCA2) was found in 3 of 19 parathyroid adenomas with aggressive clinical or histopathological features and 1 parathyroid carcinoma specimen. The significance of loss of heterozygosity (LOH) in parathyroid malignancy has been suggested by some studies. In one study, combined 1q and 11q LOH in parathyroid tumors was suggestive of malignant behavior. Others have shown that LOH at BRCA2 and Rb protein is a common event in parathyroid tumorigenesis and retention of heterozygosity seems to exclude parathyroid malignancy. This loss, which involves both the Rb gene and the BRCA2 locus on chromosome 13, suggests that tumor suppressor genes in this region other than Rb or BRCA2 may be involved in the development and progression of some endocrine tumors.
Another tumor suppressor gene that plays an important role in cell cycle regulation and its inactivation has been associated with human cancers is the p53 gene. This has also been studied in parathyroid carcinoma, but the frequency of p53 allelic loss or abnormal p53 protein expression is low in parathyroid carcinoma, and it does not appear to be a major contributor to the pathogenesis of parathyroid carcinoma. HRPT2 gene, a tumor suppressor gene on chromosome 1q25 that encodes the protein parafibromin, has been implicated in the pathogenesis of parathyroid carcinoma. The expression of parafibromin is shown to be decreased or absent in parathyroid carcinomas and the identification of inactivating germ-line mutations in HRPT2 may have significant implications for its diagnosis and management. In a recent study, 26 tumor specimens from 18 patients with adenoma and 8 patients with carcinoma were immune-stained with an antibody against parafibromin (CDC 73). Parafibromin immunostaining showed strong positivity in 17 of 18 adenomas and 2 of the carcinomas. Negative staining was noted in 3 of 8 carcinomas, and weak positivity was found in 3 of 8 carcinomas. The investigators concluded that the loss of parafibromin expression (negative or weak positivity) demonstrated 94.4% specificity in the diagnosis of parathyroid carcinomas. HPRT2 mutations are observed in 15% to 100% of the carcinomas and 0% to 4% of the adenomas. Although tumor-specific chromosomal gains and losses have been shown in adenoma and carcinoma, the hypothesis that carcinoma may arise from adenoma has not been proven. Additionally, despite reports of carcinoma in cases of MEN1, loss of regions commonly lost in adenomas, such as the 11q region associated with MEN1, is not seen in the carcinomas. Identification of the molecular markers associated with the pathogenesis of parathyroid carcinoma is essential in improving the diagnostic challenges and may serve as vital therapeutic targets in the management of this disease.
Histopathology
Two cardinal features for the diagnosis of any carcinoma are local invasion and metastasis. Some investigators believe that an unequivocal diagnosis of parathyroid carcinoma should be restricted to those tumors that invade adjacent soft tissues, thyroid gland, blood vessels, or perineural spaces or to those cases with documented metastases ; however, this may underestimate the diagnosis of carcinoma and histologic criteria may be useful in making this challenging diagnosis. Clinical and gross features of the parathyroid tumor at the time of surgical resection can significantly aid in the diagnosis of carcinoma. The size of tumor has been reported to range from 0.75 cm to larger than 6 cm. Parathyroid carcinoma often appears adherent to the surrounding tissue and is poorly circumscribed. Grossly, carcinomas are firm and grayish white compared with adenomas that are usually soft and tan colored. In one series, extent of local invasion was reported with ipsilateral thyroid invasion being the most common in 89% of the cases followed by strap muscles (71%), ipsilateral recurrent laryngeal nerve (26%), esophagus (18%), and trachea (17%).
Histologic features of lesions may be used in diagnosis of carcinoma before infiltration and metastasis has occurred. This approach has presented with difficulty in diagnosis of parathyroid carcinoma with no single feature being pathognomonic of malignancy. In making the diagnosis of carcinoma, some investigators adopt histologic criteria similar to those described by Schantz and Castleman in 1973. In studying 67 cases, they observed certain histologic features that distinguished parathyroid carcinoma from adenoma. These features were fibrous capsule or fibrous trabeculae, or both, a trabecular or rosettelike cellular architecture, the presence of mitotic figures, and capsular or vascular invasion. Similar to other investigators, however, they emphasized the importance of considering the overall picture rather than any single criterion. In one study, presence of vascular or capsular invasion and fibrous trabeculae were the most common indicators of malignancy. Some investigators consider high mitotic rate as an important feature of the diagnosis, whereas other investigators express reservation regarding the significance of vascular and capsular invasion, and feel that mitotic activity alone has limited value in diagnosis of malignancy because high mitotic rate is also observed in benign disease ( Fig. 1 ).
Although ultrastructural features of parathyroid carcinoma have been described, in establishing the diagnosis of malignancy, electron microscopic investigation did not add significantly to light microscopy. In almost all cases of carcinoma, the microscopic features were those of the chief cells. Flow-cytometric analysis of parathyroid tumors using fresh material confirms that parathyroid carcinomas are more apt to be aneuploid than are adenomas, and determination of the DNA ploidy pattern is a valuable adjunct to the histologic diagnosis of parathyroid carcinoma. Additionally, special staining, such as Ki-67, may be beneficial in diagnosis of parathyroid carcinoma.
Staging
Because of the rarity of this disease, the American Joint Committee on Cancer has not yet developed a TNM staging for parathyroid carcinoma. Hundahl and colleagues, in their review of 286 parathyroid carcinoma cases, reported an average tumor size of 3.3 cm and reported that lymph node status and tumor size did not appear to have a significant prognostic impact. These data have been questioned owing to lack of available information from the reviewed NCDB patient information; however, other reviews have found the presence of lymph node metastasis to be prognostically significant. Shaha and Shah, based on the information provided by the various reviews, proposed a staging system based on the size of the tumor, extent of local invasion, and presence of regional nodal disease and distant metastasis.
Clinical Presentation and Features
The observation that malignant parathyroid disease has a different presentation than the common benign forms of primary hyperparathyroidism has been made by many investigators. The overwhelming majority of parathyroid cancers are functioning tumors. Thus, many clinical symptoms of parathyroid cancer are similar to the benign primary hyperparathyroidism, such as weakness, fatigue, nervousness, depression, weight loss, bone disease, abdominal pain, nephrolithiasis, pancreatitis, and peptic ulcer disease. These symptoms usually manifest before local or regional invasion by the tumor. The challenge for the physician remains to differentiate between hyperparathyroidism attributable to benign disease, which is much more common than parathyroid carcinoma. Renal and skeletal involvement is a prominent manifestation of parathyroid carcinoma. Skeletal involvement, such as bone pain, osteopenia, osteoporosis, osteofibrosis, and pathologic fractures, are observed in up to 90% of the patients, and renal involvement, such as nephrolithiasis and renal insufficiency, are seen in up to 80% of the patients. The skeletal involvement is commonly observed in the spine or long bones, followed by diseases in the hands and skull and consisting mainly of subperiosteal erosions. Concomitant renal and bone involvement is reported in nearly 50% of the patients.
In most patients with parathyroid carcinoma, serum calcium and PTH are elevated, similar to benign primary hyperparathyroidism; however, the average serum calcium level for patients with parathyroid carcinoma is higher than that reported in patients with parathyroid adenoma. The serum calcium levels in patients with parathyroid carcinoma are usually higher than 14 mg/dL, and mean serum calcium levels as high as 16 mg/dL and serum PTH levels as high as 10-fold to 15-fold higher than the normal range have been reported. In a recent population study, a PTH level 10 times or more than the upper normal limit had a positive predictive value of 81% for parathyroid carcinoma. For this reason, hypercalcemic crisis is not uncommon in parathyroid carcinoma and is observed in 8% to 12% of patients. One should be cautious of using any single preoperative laboratory value in diagnosis of carcinoma, however. Additionally, levels of alkaline phosphatase and α and β subunits of human chorionic gonadotropin may be higher in patients with carcinoma than those with primary hyperparathyroidism.
Physical findings of palpable neck mass and recurrent laryngeal nerve paralysis, which are rare in benign disease, have been associated with carcinoma. Presence of palpable neck mass has been observed in up to 70% of patients with parathyroid carcinoma at presentation and in conjunction with hyperparathyroidism is suspicious for parathyroid carcinoma. Clinical presence of lymph node metastases, local invasion, and distant metastases are the sine qua non of parathyroid carcinoma. Local invasion was observed in up to 67% of patients at the time of diagnosis in one study. Nonfunctioning carcinomas are quite rare, and usually present with signs and symptoms of local growth and invasion, such as neck mass, hoarseness, and dysphagia, making this diagnosis even more challenging. Selected differentiating clinical and histologic characteristics of primary hyperparathyroidism and parathyroid carcinoma are presented in Table 1 .
| Primary Hyperparathyroidism | Parathyroid Carcinoma | |
|---|---|---|
| Female: Male ratio | 3–4:1 | 1:1 |
| Average age | 5th to 6th decades | 4th to 5th decades |
| Serum calcium (mg/dL) | ≤14 | ≥ 14 |
| Parathyroid hormone | Mildly elevated | Markedly elevated |
| Local recurrence | Rare | Common |
| Nodal involvement | No | Common |
| Distant metastases | No | Occasional |
| Palpable neck mass | Uncommon | Frequent |
| Symptoms | Mostly asymptomatic | Commonly symptomatic |
| Renal involvement | Less common | More common |
| Skeletal involvement | Less common | More common |
| Concomitant renal and skeletal involvement | Rare | Common |
| Fibrosis | Variable | Common |
| Inflammation | Infrequent | Common |
| Necrosis | Rare | Common |
| Capsular invasion | Infrequent | Common |
| Vascular invasion | Infrequent | Common |
| Atypia | Infrequent | Common |
| Ploidy | Variable | Commonly aneuploid |
| Mitosis | Rare | Common |
| HRPT2 mutation | Infrequent | Common |
Imaging studies and diagnosis
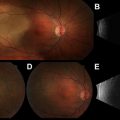
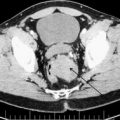
The same noninvasive imaging studies used for the diagnosis of benign parathyroid disease may be used in aiding the initial diagnosis, and in identifying recurrence and extent of parathyroid carcinoma. Ultrasonography, sestamibi scanning, computed tomography (CT), single-photon emission CT, magnetic resonance imaging (MRI), and positron emission tomography have been used for the initial diagnosis and to detect the recurrence of parathyroid carcinoma ( Figs. 2 and 3 ). Ultrasound is a noninvasive and useful study to localize primary and locally recurrent disease. Typically, parathyroid carcinomas are lobulated, hypoechoic, and relatively large, with ill-defined borders compared with adenomas. In a retrospective study, 69 patients with parathyroid lesions larger than 15 mm were evaluated by ultrasound. A high positive predictive value (PPV) for cancer was identified for infiltration (PPV 100%) and calcification (PPV 100%), and a high negative predictive value (NPV) was found for the absence of suspicious vascularity (NPV 97.6%), a thick capsule (NPV 96.7%), and inhomogeneity (NPV 100%). The investigators concluded that in parathyroid lesions larger than 15 mm, ultrasonography for specific features provides a valuable tool to identify parathyroid cancers before surgery. Other investigators, however, have pointed out that it could be challenging to distinguish a large adenoma from a carcinoma by ultrasound. Additionally, ultrasound may be used to guide fine-needle aspiration (FNA) biopsy when regional nodal metastasis is suspected. Technecium-99 m sestamibi scanning is widely used to localize parathyroid tumors. Sestamibi can also be used to detect primary parathyroid cancer or recurrent disease; however, no specific characteristics exist for distinguishing benign disease from parathyroid carcinoma. Other anatomic studies, such as MRI and CT scan, could be used for localizing recurrence or metastasis, such as in bone and liver. In a retrospective review, the sensitivity of scintigraphy, CT, and ultrasonography in detecting recurrent disease was 86%, 79%, and 100%, respectively. The addition of imaging studies to clinical presentation and the biochemical values as discussed previously may raise the suspicion of parathyroid carcinoma. Once this diagnosis is suspected, FNA biopsy of the primary lesion should be avoided, as it has been associated with tumor seeding of the biopsy tract. Furthermore, FNA cytology will not be able to distinguish benign from malignant parathyroid tumor of the primary lesion. FNA biopsy may be used to help distinguish thyroid from parathyroid tissue or identify metastatic parathyroid carcinoma. Intraoperatively, in the absence of gross local invasion or regional metastasis, the diagnosis of carcinoma can be difficult. Frozen-section analysis is also of little value and unreliable, as the histopathological features of carcinoma may overlap with those of parathyroid adenoma. In the study by Hundahl and colleagues, 86% of patients did not receive the diagnosis of carcinoma intraoperatively, even with experienced parathyroid surgeons.
Management
Parathyroid carcinoma is a slow-growing but aggressive malignant tumor and most patients die of the metabolic complications of hypercalcemia. Thus, the goals of treatment of the primary lesion, local recurrence, or metastatic disease should be to eliminate all demonstrable disease when possible and to control the metabolic complications of hyperparathyroidism. Other treatments, however, such as chemotherapy, embolization, and radio frequency ablation, have been attempted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree