© Springer International Publishing Switzerland 2016
David S. Cooper and Cosimo Durante (eds.)Thyroid Cancer10.1007/978-3-319-22401-5_99. Papillary Thyroid Cancer with Central Neck Lymph Node Metastases
(1)
Toronto General Hospital, 200 Elizabeth Street, 12 Eaton North, Room 212, Toronto, ON, Canada, M5G 2C4
Keywords
Thyroid cancerPapillary thyroid cancerCervical lymph node metastasesMicropapillary thyroid cancerCancer stagingThyroidectomyNeck dissectionRadioactive iodine treatmentCancer recurrenceThyroglobulin antibodyAbbreviations
TSH
Thyroid-stimulating hormone concentration
micro-PTC
Micropapillary thyroid cancer
RAI
Radioactive iodine
Case Presentation
A previously healthy 30-year-old female was seen by her family physician for a general medical exam. As part of this evaluation, she underwent measurement of thyroid-stimulating hormone (TSH) concentration and a neck ultrasound. It was not clear why the ultrasound was ordered, as the patient was not aware of any abnormality in her thyroid exam. The patient had no compressive symptoms (i.e., no hoarseness, dysphagia, nor dyspnea). There was no family history of thyroid cancer, nor thyroid disorders. The patient had no significant history of head and neck radiation exposure.
Diagnosis/Assessment
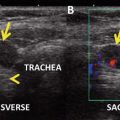
For the case presented, the baseline TSH level was normal (2.49 mIU/L) and a neck ultrasound showed a left-sided solid, 1.0 cm hypoechoic thyroid nodule with smooth margins and no microcalcifications, and there were no other thyroid nodules and no enlarged/suspicious lymph nodes. Ultrasound-guided fine-needle aspiration biopsy of the thyroid nodule was positive for papillary thyroid cancer. She underwent total thyroidectomy, and the intraoperative detection of an enlarged a paratracheal lymph node prompted therapeutic left pretracheal and left paratracheal nodal dissection as part of the same procedure. The final surgical pathology showed multifocal micropapillary thyroid cancer (micro-PTC, follicular variant), with two foci, measuring in maximal diameter, 0.9 cm (in the left lobe) and 0.2 cm (in the right lobe), respectively. There was no extrathyroidal extension of the primary tumor, with no lymphatic, vascular, nor capsular invasion. The resection margins were clear. There was evidence of chronic lymphocytic thyroiditis. Upon examination of the eight resected central neck lymph nodes, two of them were positive for PTC, measuring 8 mm and 3 mm, respectively, in maximal diameter, with no evidence of any extranodal extension.
The clinicopathologic stage of disease in this case was interpreted as follows:
Postoperatively, the patient started taking levothyroxine and recovered uneventfully, with normal calcium and parathyroid hormone levels and no problems with her voice. Approximately 11 weeks following surgery, while on levothyroxine therapy (TSH 7.24 mIU/L with a normal free thyroxine concentration), the thyroglobulin was measured to be <0.9 ng/dl, but thyroglobulin antibodies were present at a level of 97 IU/L (thyroglobulin antibody reference range <39 IU/L and the assay detection limit 20 IU/L). Given the presence of thyroglobulin antibodies, the thyroglobulin measurement was considered unreliable, due to potential assay interference [5]. A postoperative ultrasound of the neck 12 weeks after surgery was negative. Her levothyroxine dose was increased, with the intention of suppressing the TSH concentration (<0.1 mIU/L). The patient’s endocrinologist recommended radioactive iodine (RAI) adjuvant treatment, given the presence of nodal metastases associated with increased risk of disease recurrence; another reason why RAI was recommended was to facilitate disease follow-up. However, the patient indicated that she did not want to take RAI, unless there was proof that it could reduce the risk of dying from thyroid cancer or distant metastatic recurrence, specifically in her situation. She strongly disliked the idea of taking any form of “radiation,” unless it was clear that it could prevent death or distant metastases (which were her primary concerns). She was then referred an endocrinologist at a tertiary care center for further counseling.
Literature Review
Classification of Lymph Node Disease
Randolph et al. of the ATA Surgical Affairs Committee have reviewed the literature on prognostic significance of nodal metastases of papillary thyroid carcinoma and have proposed a categorization system for nodal disease [6]. Pathologic N1 (pN1) is any metastatic papillary thyroid cancer detected on the pathologic specimen of any resected lymph nodes [6]. Clinically apparent nodal disease , referred to as clinical N1, (cN1) is defined as metastatic lymph nodes identified on either physical examination, diagnostic imaging studies, or intraoperative inspection by the surgeon, and the absence of such features is clinical N0 (cN0) [6]. The presence of one or more metastatic lymph nodes that are visible on preoperative physical examination, ultrasound, or during surgery (the latter according to surgical and pathologic reports) has been independently associated with increased risk of recurrence of disease in a multivariable analysis, including data from 545 patients [7]. However, in the central neck, the accuracy of intraoperative surgical inspection is only about 60 % [8], with larger affected nodes being more readily clinically detected [9]. Moreover, the significance of subclinical, low-volume nodal disease in the central neck is not clear.
Regardless of how cN1 disease is detected, resection of such affected nodes and relevant nodal compartments is termed “therapeutic neck dissection ” [6]. In contrast, “prophylactic neck dissection ” is defined as nodal dissection in the absence of any evidence of cN1 disease prior the procedure [6]. Randolph et al. divided nodal disease into two categories: lower-risk N1 disease and higher-risk N1 disease [6]. Lower-risk N1 disease has been defined by the presence of the following criteria: (a) clinical N0; (b) low-volume nodal disease, specifically micrometastatic nodes (i.e., largest node <0.2 cm in diameter) or small nodal metastases (0.2 to <1.0 cm in diameter); and (c) ≤5 small lymph node metastases (i.e., each measuring <1.0 cm in diameter) [6]. Higher-risk N1 disease has been defined by the presence of the following criteria: (a) clinically detectable lymph node metastases (cN1), (b) metastatic lymph node(s) >3 cm, and (c) >5 metastatic lymph nodes [6]. Randolph et al. have also reported that gross extranodal extension, increasing number of metastatic lymph nodes with microscopic extranodal extension, or the combination of microscopic extranodal extension and metastatic lymph nodes >1 cm is also predictive of a higher risk of disease recurrence [6]. However, the predictive performance of the ATA metastatic lymph node classification system from Randolph et al. [6] has not yet been independently validated. It is important to note that according to the TNM/AJCC VII system, pathologic N1 disease is categorized as follows: N1a (metastases to the pretracheal, paratracheal, and/or prelaryngeal or Delphian lymph nodes, which are in the central neck) and N1b (metastases to unilateral, bilateral, or contralateral cervical or superior mediastinal nodes) (i.e., including the lateral neck or mediastinum) [1, 2]. The location of nodal disease has been reported to be associated with the size of involved nodes in papillary thyroid cancer, particularly for bulky enlarged nodes. For example, Chow et al. reported that 13 % of N1a and 56 % of N1b papillary thyroid cancer patients had involved nodes >2 cm in diameter (p < 0.001) [10]. Ito et al. suggested that for papillary thyroid cancer patients whose nodal disease is detected on preoperative imaging, the cause-specific survival of patients with N1b level nodal involvement is not significantly different from that of those with N1a nodal involvement [11]. However, in this study [11], the disease-free survival was adversely affected in papillary thyroid cancer patients with N1b level nodal involvement who had pathologic evidence of aggressive nodal disease, including the lymph nodes measuring >3 cm in diameter, extranodal extension, or ≥5 or more involved nodes [11]. Furthermore, the presence of two or more such adverse features in N1b disease was associated with reduced cause-specific survival [11]. Age also appears to be an important prognostic variable in N1 disease. For example, Verberg et al. reported that in differentiated thyroid cancer, patients aged ≥45 years who have lateral neck lymph node metastases have a reduced long-term life expectancy, but life expectancy is not significantly impacted in younger patients with similar disease features [12]. Furthermore, Hughes et al. have reported that in differentiated thyroid cancer patients with N1 disease, the recurrence rate was 8 % in those <45 years of age, as compared with 31 % in those ≥45 years of age [13]. In this study, all disease recurrences were successfully treated in the N1 patients aged <45 years, but only about a third of those aged ≥45 years of age [13]. In conclusion, the size and number, location of metastatic lymph nodes, the presence of extranodal extension, and patient age are relevant considerations in risk stratification of N1 disease.
Epidemiology of N1 Disease in Patients with Micro-PTC
Lymph node metastases are evident at the time of diagnosis in approximately 12–64 % of cases of micro-PTC [14–26]. If nodal metastases are present in this situation, the ipsilateral paratracheal compartments, followed by the pretracheal compartments, are the levels most frequently affected [20]. Lateral neck nodal metastases may be present in about 3–7 % of individuals with micro-PTC [15, 20, 24, 27, 28]. The presence of primary tumor extrathyroidal extension [14, 19, 29] and tumor multifocality [19, 29, 30] are risk factors for the presence of lymph node metastases with micro-PTC. In summary, N1 disease is not uncommon in patients with micro-PTC, and if it is present, it is most frequent in the central neck.
Prognosis of N1 Disease in Patients with Micro-PTC
The overall risk of disease recurrence of persistence in patients with papillary thyroid microcarcinoma and positive lymph nodes (without distant metastases at presentation) has been reported to range between 3.0 and 22 % [15–17, 19]. The risk of dying of thyroid cancer and developing distant metastatic recurrence in this context are important considerations. In a recent retrospective review of micro-PTCs, Mercante et al. reported that in a subgroup of 27 patients with T1aN1a disease, none of the patients died of disease nor developed distant metastases (follow-up about 8 years) [19]. Similarly, Kim et al. found that in a subgroup of 168 individuals with micro-PTC with no evidence of macroscopic extrathyroidal extension nor distant metastases at initial presentation, no patient died of thyroid cancer nor developed distant metastatic disease (mean follow-up about 5 years) [15]. In a retrospective review of micro-PTC cases from a hospital in Hong Kong, Chow et al. [22] reported that in a subgroup of 48 patients with micro-PTC and various degrees of severity of lymph node disease without distant metastases, 2 % died due to thyroid cancer (1/50) and 4.0 % (two patients) developed distant metastatic recurrence (mean follow-up about 8 years) [22]. In a retrospective chart review of patients with differentiated thyroid carcinoma ≤1 cm in diameter treated in the years 1962–1995 in France, Baudin et al. reported that in node-positive micro-PTC patients without distant metastases at primary presentation, none of the patients died from thyroid cancer (0/113), and only 1 % developed distant metastatic recurrence (1/113) (mean follow-up of about 7 years) [21]. Also, in a retrospective review of micro-PTC cases at the Mayo Clinic, Hay et al. studied that the outcomes of a subgroup of 273 patients had positive lymph nodes at diagnosis [17]. Hay et al. indicated that no female with initial disease confined to the neck ultimately died of disease or developed distant metastases, but one male with extensive bulky lateral neck disease at presentation developed bone metastases and died of the disease, approximately 30 years after presentation (mean study follow-up 17 years) [17]. In summary, in papillary thyroid microcarcinoma patients with limited nodal involvement, no evidence of other adverse disease features, and no distant metastatic disease at the time of presentation, the risk of dying of thyroid cancer is likely about 0–2 % and the risk of developing distant metastatic recurrence is likely about 0–4 %.
In patients with papillary thyroid microcarcinoma and lymph node metastases (T1aN1), the risk of local-regional recurrence of disease in the neck or lymph nodes is another relevant consideration. The risk of local-regional recurrence in T1aN1 micro-PTC has been reported to range from 3 to 16 % [15, 17, 21]. Furthermore, the incidence rate of local-regional recurrence has been subdivided according to the level of nodal involvement at the time of initial diagnosis as follows, in two of the more recent studies: N1a (central neck)—0 to 3 % and N1b (lateral neck or mediastinum)—2 to 11 % (excluding individuals with extrathyroidal extension of the primary tumor at initial diagnosis) [15, 19]. Based on these limited data, it appears that nodal recurrence of disease is relatively uncommon in patients with micro-PTC whose initial nodal disease is confined to the central neck, in the absence of other adverse disease characteristics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree