MDACC
AHPBA/SSO/SSAT
NCCN
Resectable
Celiac axis
No extension
Clear fat plane
No contact
Common hepatic artery
No extension
Clear fat plane
No contact
Superior mesenteric artery
Clear fat plane
Clear fat plane
No contact
Portal-superior mesenteric vein
Abutment or encasement (no occlusion)
No abutment, distortion, encasement, or tumor thrombus
No contact; ≤180° contact without vein contour irregularity
Borderline resectable
Celiac axis
Abutment
No abutment or encasement
Contact ≤180° or contact >180° with uninvolved GDA
Common hepatic artery
Abutment or short segment encasement
Abutment or short segment encasement
Short segment encasement or abutment without extension to CA or HA bifurcation
Superior mesenteric artery
Abutment <180°
Abutment <180°
Contact <180°
Portal-superior mesenteric vein
Short segment occlusion amenable to reconstruction
Abutment >180° or occlusion amenable to reconstruction
Contact <180° or ≥180° with vein contour irregularity
Locally advanced
Celiac axis
Encasement
Abutment or encasement
Contact >180°
Common hepatic artery
Encasement with no reconstruction option
Encasement with extension to celiac axis
Contact with extension to celiac axis or bifurcation
Superior mesenteric artery
Encasement >180°
Encasement >180°
Contact >180° or contact with first jejunal branch
Portal-superior mesenteric vein
Occluded with no reconstruction option
Occluded with no reconstruction option
Unreconstructible
Accurately identifying patients with BR or LA tumors and predicting who will require vascular resection and reconstruction is essential to the development of appropriate treatment regimens. Currently, pancreas-protocol multiphase CT is the most widely used imaging tool to characterize vascular involvement. In fact, CT evaluation of the tumor–vein interface has been shown to be highly predictive of the need for vascular resection [12]. In general, cross-sectional imaging should be obtained prior to endoscopy, biliary stenting, or tissue biopsy in order to prevent peripancreatic inflammation that can obscure the tumor–tissue interface and decrease the imaging sensitivity [13]. In addition to assessing major vascular involvement, regional arterial and venous anatomy should be thoroughly examined. This review should include inspection of the first order jejunal and ileal branches of the SMV, precise location of IMV insertion, and evaluation of hepatic artery variants that will influence the operative strategy [14, 15].
9.3 Venous Resection
Given its intimate relationship to the PV–SMV, venous involvement by PDAC of the head and uncinate is a frequent occurrence. All pancreas surgeons should be capable of performing standard venous resections and reconstructions given their relative frequency and the inability to accurately predict venous involvement preoperatively. Although initially felt to be associated with a high risk of complications, most contemporary series of PD with concomitant venous resection have shown the procedure to be safe and allow a larger proportion of patients to benefit from a margin-negative resection. Indeed, as many as 50% of PDs performed today at large referral centers require some type of vascular resection. Some investigators have even proposed routine segmental venous resection at the time of PD regardless of venous involvement in order to ensure a wide negative margin; however, such a policy is not supported by existing data [16].
9.3.1 Technical Considerations
The two most important considerations for venous resection and reconstruction are the location and the extent of tumor involvement of the vessel. Broadly, the location of tumor involvement can be one of three locations: PV above the confluence, involving the PV–SMV at the confluence, and SMV below the confluence. Various classification systems exist to describe the extent of tumor involvement as a means of guiding the reconstruction. Tseng et al. have proposed a classification system that takes into account both the extent of tumor involvement and its relation to the portosplenic confluence (Fig. 9.1) [18]. The International Study Group of Pancreatic Surgery (ISGPS) has also proposed a simple classification system [19]. In general, the limits of venous resection extend proximally to the bifurcation of the left and right PV and distally to the first jejunal/ileal branches of the SMV at the root of the mesentery. Technically, one of the principal jejunal or ileal branches may be sacrificed as long as the other can be maintained patent. However, vascular dissection, obtaining vascular control, and creation of anastomoses in the root of the mesentery are both challenging and perilous. Ensuring that safe proximal and distal vascular control is achievable is critical prior to embarking on vascular dissection.
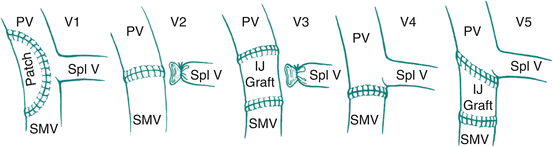

Fig. 9.1
Classification of portal vein–superior mesenteric vein reconstruction according to location and extent of required resection (Used with permission [17])
Although a thorough operative plan should be established prior to entry to the operating room based on a critical examination of cross-sectional imaging, formal assessment of the tumor–vessel interface may be performed following transection of the pancreas and acquisition of complete venous isolation and control. When tumor involves the right lateral wall of the vein, the tumor may be excised with an en bloc segment of vein. Lateral venorrhaphy may be performed with either direct suture closure (if less than approximately 25% of the wall is involved) or patch closure using either autologous vein graft or bovine pericardial patch. For more extensive venous involvement, segmental resection of the vein should be performed. A thorough attempt should be made at primary anastomosis whenever possible. Defects as long as 4 cm or so can often be overcome after removal of the specimen and full mesenteric mobilization. In cases in which primary anastomosis is not possible, an interposition graft should be used. Autologous grafts are preferred over synthetic grafts due to the risk of infection and thrombosis [20]. Our preference is to use the left internal jugular vein for interposition grafts due to its favorable size match to the PV–SMV; however, left renal vein, saphenous vein, and deep femoral vein have also been employed [21]. Alternatively, a customized bovine pericardial tube interposition graft can be fashioned using a vascular stapler.
Since the SMA margin is the margin most frequently found to be positive after PD for PDAC, a fundamental technical principle is to maximize the clearance of cancer cells from the retroperitoneum by meticulous dissection along the periadventitial plane of the SMA. Since tumor involvement of the PV–SMV interferes with straightforward exposure and dissection of the proximal SMA, the conduct of the dissection must be adjusted accordingly to overcome this limitation. For tumors that do not involve the PV–SMV, the vein can typically be reflected to the patient’s left after separation from the tumor and pancreas in order to expose the SMA. Since this is not possible when the pancreatic tumor involves the venous confluence, we often recommend division of the splenic vein which permits rightward mobilization of the PV–SMV (Fig. 9.2a). The SMA dissection then begins inferiorly at the level of the first jejunal branch of the SMV and proceeds cephalad. All fat, fibrous tissues, lymphatics, and nerves are dissected towards the right and the inferior pancreaticoduodenal arteries are individually ligated. The pancreatic neck is divided and the dissection of the SMA, avoiding circumferentially skeletonizing the vessel, is continued towards its origin on the aorta. Once the deep retroperitoneal tissues are divided, the tumor will remain attached only to the PV–SMV and venous reconstruction can be performed (Fig. 9.2b).
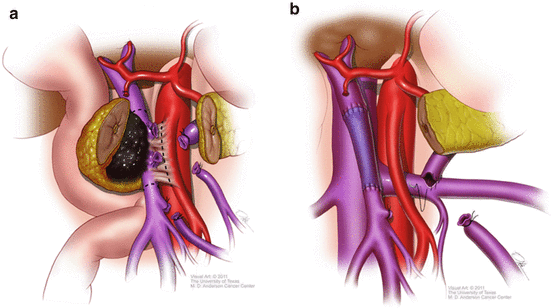

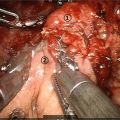
Fig. 9.2
(a) Division of the pancreas and splenic vein optimizes exposure of the SMA in cases of tumor involvement of the portosplenic confluence. Dotted line demonstrates correct line of dissection adjacent to the SMA. (b) Once SMA dissection is complete, venous resection and reconstruction may be performed. Splenorenal shunt (shown here) may or may not be performed depending on the insertion of the IMV and adequate gastrosplenic outflow (Used with permission [22])
There are several key physiological issues that should be considered when undertaking venous resection during PD. Although we do not routinely perform superior mesenteric artery (SMA) clamping, others have proposed this practice as a means of preventing intestinal wall congestion and edema that might interfere with subsequent anastomosis. Equally important is the maintenance of hepatopetal flow by minimizing vascular clamping time and ensuring adequate venous patency. An underappreciated issue is the maintenance of gastrosplenic outflow when the splenic vein must be divided during venous segmental resection. In the majority of cases, outflow is maintained through retrograde collaterals such as the inferior mesenteric artery (IMV) or coronary vein. However, while the IMV typically inserts into the splenic vein, it may insert into the SMV below the confluence in up to one-third of patients. In these cases, splenic vein division results in inadequate gastrosplenic outflow, especially if the coronary vein has been divided. In order to prevent sinistral hypertension, a distal splenorenal shunt may be created [23]. Finally, in patients with SMV–PV occlusion and cavernous transformation, pancreatic resection and venous reconstruction are associated with significant venous collateral hemorrhage. In these situations, creation of a temporary mesocaval shunt prior to pancreatic or portal dissection may result in decompressed varices and a safer dissection.
9.3.2 Outcomes
Venous resection and reconstruction is a technically challenging endeavor and one might expect a higher rate of perioperative complications. Indeed, an analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) found higher postoperative morbidity (39.9% vs 33.3%) and mortality (5.7% vs 2.9%) rates in patients undergoing PD with vascular resection compared to standard PD [24]. However, the dataset is limited in its ability to accurately code these events; for example, operations with inadvertent vascular injury and repair, typically associated with higher postoperative complication rates, were not excluded. Other single institution series [25–27], multi-institutional reports [8], and meta-analyses [7, 28, 29] have demonstrated no statistically significantly different morbidity and mortality rates of patients undergoing concomitant venous resection with PD.
It is now widely accepted that venous involvement should not be a contraindication to resection on the basis of oncologic reasons. In fact, pancreatectomy with venous resection results in improved survival compared to nonoperative therapies. Lygidakis et al. randomized patients with limited vascular involvement to complete surgical resection or double bypass and found 2-year survival rates of 81.8% vs 0%, respectively [30]. In a non-randomized prospective, multi-institutional study comparing pancreatectomy with vascular resection versus chemoradiation alone, median survival in the surgery group was 11.8 months longer [31]. Several contemporary reports have suggested comparable long-term survival for patients undergoing PD with concomitant PV–SMV resection compared to PD alone, especially when neoadjuvant therapies are utilized [32, 33].
In general, porto-mesenteric venous reconstruction during PD is associated with high patency rates. Primary repair and autologous vein grafts are associated with the lowest rates of thrombosis [34, 35]. Although venous thrombosis can occur any time after surgery, acute thrombosis in the immediate postoperative period is associated with the greatest morbidity. Late thromboses are often related to cancer recurrence [36, 37]. Although the optimal pharmacologic prophylaxis is not well established, we typically recommend aspirin for patients who have undergone venous reconstruction. This is administered on the first postoperative day and patients are encouraged to remain on aspirin life-long. Patients also receive subcutaneous lovenox for 28 postoperative days at a prophylactic dose, as do all patients who undergo pancreatectomy.
9.3.3 Case Example
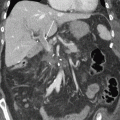
A 52 yo woman presented with PDAC in the head of the pancreas involving the portosplenic confluence with short segment occlusion (Fig. 9.3a). She underwent preoperative therapy consisting of 2 months of mFOLFIRINOX and 50.4 Gy of external beam radiation. Restaging scans demonstrated no progression of disease and laparoscopy did not reveal peritoneal or hepatic metastases. At the time of surgery, the tumor was found to be involving the anterior pancreas invading through neck into the portosplenic confluence. The neck of the pancreas and the splenic vein were carefully divided. With the tumor and PV–SMV retracted towards the right, a meticulous SMA dissection was performed as described above. Finally, segmental vein resection was performed en bloc with PD; reconstruction was performed with a primary vein anastomosis (Fig. 9.3b). Because the IMV inserted into the splenic vein, thereby providing adequate collateral outflow, a splenorenal shunt was not felt to be necessary.
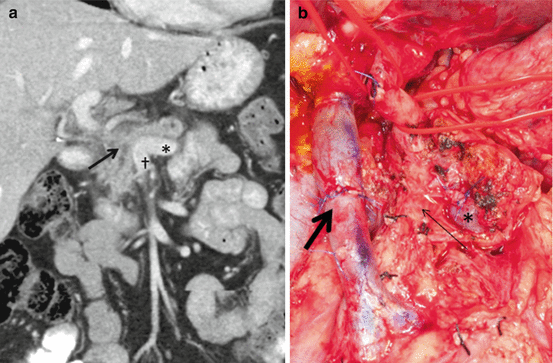

Fig. 9.3
(a) Example of pancreatic cancer (arrow) with complete encasement of the PV at the portosplenic confluence where the splenic vein (asterisk) joins the SMV (dagger). (b) Division of the splenic vein (asterisk) allows adequate exposure of the SMA (thin arrow) for complete dissection followed by PV–SMV resection and primary anastomosis (thick arrow)
9.4 Arterial Resection
Traditionally, arterial resections for pancreas cancer have not widely been supported because these operations have been associated with significant perioperative morbidity, high rates of positive resection margins, and an unclear survival benefit [9]. In addition, arterial invasion is generally considered to be a marker of aggressive tumor biology and the presence of occult metastatic disease. However, with improvements in effective preoperative therapy, vascular resection techniques, and perioperative care, aggressive operations with arterial resections are more frequently being performed. In fact, isolated common hepatic artery involvement and SMA abutment both qualify as BR disease (not unresectable) in current staging systems (Table 9.1). Nevertheless, acknowledging significant perioperative risk and uncertain oncologic benefits, all patients with arterial involvement should be strongly considered for preoperative therapy. Using a multimodality approach with induction chemotherapy and local irradiation of the tumor and regional lymph nodes, this strategy treats the micrometastatic disease presumed present in all patients, helps sterilize critical surgical margins and lymph nodes, and provides a critical selection period to ensure favorable tumor biology and personal physiology prior to major surgery. Only after completing aggressive, standardized preoperative therapy, should patients be considered for pancreatectomy with concomitant arterial resection. Given the technical complexity and perioperative risk, these operations should only be offered in highly selected patients at high-volume experienced referral centers.
9.4.1 Technical Considerations
Arterial structures that are risk for local involvement by cancers of the head of the pancreas include the CA, CHA and SMA and variant hepatic artery anatomy, most commonly a replaced right hepatic artery (RRHA) that arises from the SMA posterior to the head of the pancreas. Potential vascular interventions include simple ligation, resection with primary repair, and resection with interposition grafting. Preoperative planning is essential and depends upon high quality thin-cut CT angiography to define the extent of tumor involvement, appropriate reconstruction options, and aberrant arterial anatomy.
With regard to CHA resections, the anatomic limits of potential resection are defined by the bifurcation of the left and right HAs distally and the CA proximally. Reconstruction to distal segments of the left/right HA are technically challenging given their small size and are at high risk for anastomotic failure, the consequences of which include bilioenteric anastomotic leak, hepatic ischemia, and liver abscess. Arterial resections may extend proximally to the root of the CHA as it arises from the CA for tumors in the head of the pancreas. En bloc CA resections almost always occur in conjunction with a left-sided pancreatectomy, also known as the modified Appleby procedure, with hepatic perfusion maintained via collateral circulation through the pancreaticoduodenal and gastroduodenal vessels [38]. For CA resections, retrograde flow through the gastroduodenal should be confirmed prior to committing with the resection; if retrograde flow exists, then ligation of the CHA should have little consequence. Complex resection and vascular reconstructions involving the celiac axis (as well as left gastric and splenic arteries) have also been performed in conjunction with PD or total pancreatectomy but may require mutivisceral resection (e.g., splenectomy, gastrectomy), the degree of which is determined by the resultant arterial insufficiency [39]. After arterial resection, primary anastomosis should be attempted whenever feasible. This is typically possible when short segment CHA resections are performed. When interposition grafting is required, various conduits may be used. Our preference is the reversed saphenous vein graft (rSVG) because of its size compatibility and ease to obtain. Others have reported using superficial femoral artery (with the primary vessel reconstructed with synthetic graft) [40], internal iliac artery [41], or other visceral arteries including the splenic [42], left gastric [43], or gastroduodenal [44]. Occasionally, interposition grafting will be required to reconstruct a RRHA that was involved by tumor and resected en bloc with PD. Alternatively, preoperative embolization of the RRHA may promote collateralization and obviate the need for vascular reconstruction [45].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree