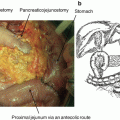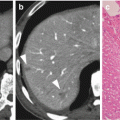
Fig. 8.2
PV reconstruction type 2. Tangential resection and reconstruction by using a peritoneal patch (white arrows). Hepatic artery with stump of the gastroduodenal artery (broken white arrow), pancreatic remnant (black star)
Type 3 and type 4 of reconstruction imply a segmental resection, resulting in a longer defect of the venous axis. For all attempts of these resections, the mesenteric root should be mobilized completely by resolving the attachment of the right hemicolon to the retroperitoneal adhesions (Cattell-Braasch manoeuver [20]). This allows to bridge even long distances after resection of the vein and graft interposition (type 4) can often be avoided.
In type 3 resections, SMV/PV continuity is restored by a direct end-to-end anastomosis (Fig. 8.3). In case of corresponding diameters of the distal and proximal lumen, there is no obstacle for free venous drainage of the bowel. If it is not possible to bridge the resected length by approximation of the proximal and distal vessel lumen and performing a direct anastomosis, a vascular graft needs to be inserted. For this type 4 reconstruction, autologous as well as allogenous grafts can be used. Regarding autologous grafts, there are various possibilities (Table 8.1). Most commonly, the saphenous vein, the left renal vein (in case of patency of the ipsilateral ovarian/testicular vein to preserve kidney drainage), or the internal jugular vein have been described (Table 8.1, [21–33]). All of these grafts imply that a harvesting procedure must be performed and that the respective vessel is available and suitable with respect to the diameter and to the length which is needed for the reconstruction. As described before, also a peritoneal patch may be harvested and used to create a tubular graft (i.e., by placing the patch around a drainage tube and form the graft by longitudinal suture [19]). All of the described techniques of autologous graft insertion have been reported with good outcomes regarding postoperative morbidity and patency [21–34]. If no autologous graft material is available or in case of unplanned venous resection, that requires an “emergency” reconstruction, synthetic material represents a valid option for reconstruction, as well [28, 30]. A ringed polytetrafluoroethylene (PTFE) graft seems preferable as this type of prosthesis offers excellent flow conditions and patency rates [30, 34, 35]. The disadvantage of inserting a synthetic graft lies in the fact that any artificial material may cause problems in case of infection or anastomotic leakage. The in situ situation of a graft in combination with a pancreatic fistula must be regarded as a high-risk constellation for arrosional bleeding or for long-lasting graft infection, which is known difficult to treat. However, in recent clinical studies, no difference in surgical outcome and long-term survival was shown when different types of venous reconstruction (venorrhaphy, end-to-end anastomosis, graft insertion) were compared [30].
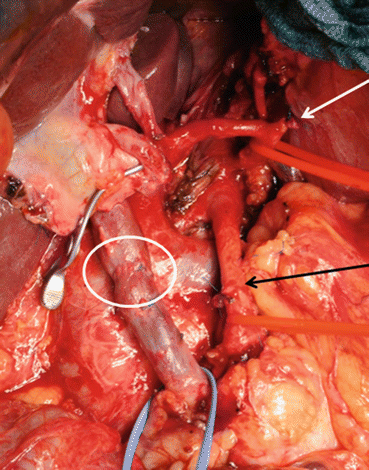

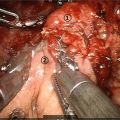
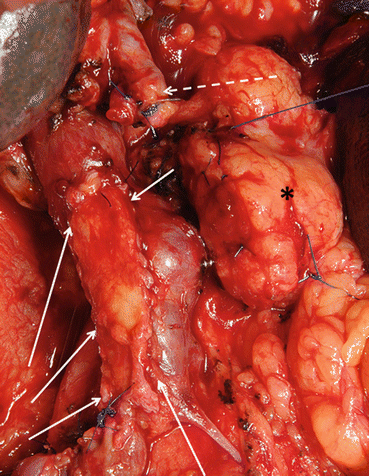
Fig. 8.3
PV/SMV resection during total PD. Type 3 reconstruction without reinsertion of the splenic vein due to concomitant splenectomy. SMV/PV anastomosis (white circle), hepatic artery with stump of the gastroduodenal artery (white arrow), completely dissected SMA (black arrow)
Table 8.1
Methods of reconstruction in type 4 venous resections
• Peritoneal patch |
• Left Renal vein |
• Gonadal vein |
• Saphenous vein |
• Internal jugular vein |
• External iliacal vein |
• Allogenous iliacal vein (deceased donors) |
• Bovine patch |
• PTFE prosthesis |
An unsolved problem is the confirmation of a R0 situation during PV/SMV resection. As the small bowel does not tolerate warm ischemia due to complete venous congestion (regardless of a synchronous clamping of the SMA) for a long time, the performance of a frozen section of the distal and proximal cut margin of the resected vein segment cannot be routinely performed. From large series, the R1 rate in this position ranges from 13% up to 50% [25, 26, 30]. Despite these considerably high rates in some studies, the oncological outcome may not be compromised and seems to be mostly determined by other factors (i.e., lymphatic spread, lymph node metastases, R1 status in other positions [16]).
Management of the splenic vein is another important issue in segmental resections of the SMV/PV [36]. If the site of resection is located clearly above or below the splenic vein confluence, this can be preserved without any changes in the physiological venous drainage of spleen and stomach. However, as the venous confluence is often the site of tumor infiltration, the proximal splenic vein is often part of the resected specimen. From the technical point of view, the splenic vein can be closed or reinserted during venous resection in PD. In case of lateral reinsertion of the splenic vein, it must be assured that no tangential tension on the venous anastomoses occurs (Fig. 8.4). This may otherwise promote narrowing and thrombosis of the respective vessels. Therefore, a closure of the splenic vein without reinsertion can be advisable in certain situations as long as this does not compromise splenic and gastric drainage which is sustained via left-sided collaterals in many patients.
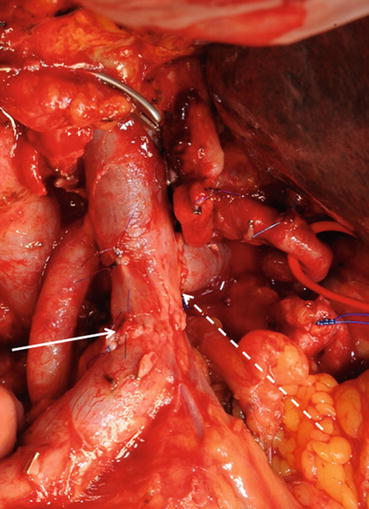

Fig. 8.4
PV/SMV resection during PD with reinsertion of the splenic vein. White arrow: SMV/PV anastomosis, broke white arrow: reinsertion of the splenic vein
Another aspect that has to be taken into account during venous resections is the perfusion of the right hemicolon. In case of removal of the middle colic vein or the ileocolic vein, which are common locations for lymph node metastases in PDAC [37], a right colectomy may be necessary to avoid colon-associated ischemic complications.
In distal pancreatectomy (DP), venous resections are less frequently performed. The technical challenge during this procedure is the fact that the remaining pancreatic head may block the approximation of the proximal and distal lumen and consequently create tension of both ends when a direct suture is attempted. Furthermore, a lateral compression of the anastomosis with a consequent thrombosis may occur. To avoid both of these situations, a segmental graft insertion is advisable to achieve best rates of patency. Due to the high risk of postoperative pancreatic fistula in DP, autologous graft material should be chosen and a synthetic graft with the inherent risk of fistula-associated infection should be avoided.
Regarding total PD with venous resections, an end-to-end anastomosis with or without graft insertion is possible and the splenic vein is generally removed as most total PDs for oncological indications include splenectomy. In this situation, the venous drainage of the stomach needs to be considered, i.e., by preserving the gastric coronary vein (see Sect. 8.4). Otherwise, an adopted resection of the stomach must be performed to avoid ischemic complications.
8.3.2 Laparoscopic/Robotic PD with Venous Reconstruction
Laparoscopic as well as robotic PD with venous reconstruction has been reported in small case series [38–42]. The respective studies have included between 11 and 34 patients for laparoscopic and robotic approaches, respectively. In all studies, surgical outcome has been proven to be comparable or even superior to open resections, especially with regard to blood loss. The disadvantages, however, include the significantly longer operation times and the excess costs, especially associated with robotic approaches. An Italian publication by Boggi et al. [39] showed that these costs account to app. 6200 Euro per procedure. Furthermore, the authors of all reports confirm that these procedures require a great expertise and are only applicable for highly selected patients. Consequently, the impact of minimally invasive PD with venous resections may increase in the future. To date, these procedures have to be regarded as highly limited approaches that are only available in specialized centers and scientific evidence for their usefulness in terms of surgical or oncological outcomes to justify the burden of excess costs is still lacking.
8.3.3 Venous Resection in Multivisceral Approaches
The resection of adjacent organs during PDAC surgery is an established procedure to achieve a radical tumor removal [43–46]. Although multivisceral resection is associated with an increased morbidity, perioperative mortality and long-term survival are not influenced in these patients. In approximately 20% of the patients, multivisceral resection is performed together with portal or superior mesenteric vein resections [43, 46]. This additional procedure does not increase the risk for complications and should therefore be performed in patients qualifying for an extended approach of complete tumor removal.
8.3.4 Combined Vascular Resections
Resection of the portal or superior mesenteric vein in combination with the celiac axis or mesenteric artery is—comparable to arterial resections alone—not a standard procedure and has only be performed in a small number of patients to date [46–48]. It may be an individual option for selected patients, especially after neoadjuvant treatment. Although it may be technically feasible, oncological outcome of these procedures is determined by the arterial tumor encasement. The oncological value of combined resection of both—portal vein and celiac axis or superior mesenteric artery—has not been proven in larger series. Furthermore, combined resections may be associated with a higher surgical morbidity and impaired postoperative quality of life than standard pancreatic operations, especially with regard to intestinal discomfort and diarrhea due to a higher risk of autonomous denervation of the small bowel during the extensive dissection of perivascular tissue including the respective nerve plexus.
8.4 Special Aspects and Limitations of Venous Resections
8.4.1 Gastric Coronary Vein
During preparation for venous resection, the gastric coronary vein may be injured accidentally or by intention in the case of tumor adherence. The coronary vein, or left gastric vein, drains both the anterior and posterior stomach walls, which results in the relevant physiological importance of this vessel for the perfusion of the stomach [49]. There are two major anatomical variations, as the junction of the coronary and portal vein may be located above or within the confluence of the splenic and portal vein (variant A) or the coronary vein may drain into the splenic vein (variant B) in a situation in which the junction may be located as far left as the junction between the splenic and inferior mesenteric vein [50]. In both situations, the coronary vein may be injured or sacrificed during portal vein resection at the level of the confluence. Especially when total PD is performed in combination with splenectomy, venous drainage of the stomach may be severely compromised, leading to venous congestion and consequent ischemia. A comparable situation can arise, when partial PD is combined with portal vein resection and the splenic vein is not reinserted, which must not be necessarily performed. Acutely compromised venous drainage of the stomach often results in visible intraoperative congestion and ischemia of the stomach. Consecutively, a resection of the stomach has to be performed or the venous drainage needs to be restored. Furthermore, long-term complications, such as left-sided portal hypertension, may occur due to impaired venous drainage of the stomach.
During PD in variant A anatomical situation, the portal vein resection may be performed below the level of the coronary–portal vein junction which preserves sufficient stomach drainage, irrespective of the splenic vein. Second, the coronary vein may be preserved in variant B anatomy when the splenic vein is reinserted into the portal vein after resection. A reinsertion of the splenic vein is not always possible and advisable, as the spleen may put tension on or twist the portal vein anastomosis, resulting in a risk of immediate or postoperative portal vein thrombosis. In this third situation, the spleen can be preserved without reconstruction of the splenic vein, if the coronary vein is reinserted, which can usually be performed without tension and ensures the drainage of both the spleen and the stomach.
In total PD for malignancies, the spleen, including its vessels, is routinely removed. In a variant A anatomical setting, this is not associated with any problems as long as the coronary vein is not injured during dissection or lymphadenectomy, and the stomach may be completely preserved. In the case of portal vein resection, including the coronary and portal vein junction, injury of the coronary vein or in variant B anatomical situations, the coronary vein is cut and the stomach is consecutively drained via the remaining cranial short gastric vessels that are connected to the esophageal drainage. Therefore, the body and fundus of the stomach are subjected to severe venous congestion. In this situation, the remaining part of the coronary vein should be reinserted to avoid intraoperative gastric resection or postoperative complications due to underestimation of this congestion.
Reinsertion of the coronary vein can be performed in an end-to-side fashion. Whenever possible, depending on the extent of resection, a patch of the splenic vein (anatomical variant B) should be preserved during preparation to enable a wider anastomosis and thereby lower the potential risk of thrombosis. The anastomosis is located above the portal vein anastomosis. As the coronary-portal vein anastomosis is the only outlet of venous drainage, the high blood flow will widen the diameter of the vessel and a thrombosis is unlikely to occur. When performing the coronary vein anastomosis, it is of utmost importance to ensure that no twisting of the vein or tension occurs. As the stomach is completely mobile, the coronary vein can always be approximated easily, even when a segment has been removed during the resection phase [51].
Restoration of stomach drainage has to be evaluated intraoperatively after completion of the reinsertion before completing the gastrointestinal reconstruction. In the postoperative period, a direct control of patency of the coronary vein is not possible. In case of suspected occlusion and consecutive gastric perfusion problems, an endoscopic control of the stomach is the examination of choice to evaluate potential ischemia.
Impairment of gastric drainage can result in two postoperative clinical scenarios, either an immediate intraoperative ischemia with the ultimate consequence of a potentially subtotal or even total gastrectomy, or in a delayed postoperative stomach perfusion failure. The latter may lead to either revision with stomach resection or—if less pronounced—to a long-lasting delayed gastric emptying. Both of these problems can be avoided if careful attention is paid to the coronary vein and a reinsertion is performed when necessary.
As subtotal or even total gastrectomy combined with partial or total PD is associated with an impairment of the patients’ quality of life and may be associated with increased perioperative morbidity [46], surgical approaches should aim to preserve the stomach, unless a resection is required for oncological reasons. Another aspect besides acute perfusion failure of the stomach is the development of chronic left-sided portal hypertension following splenic vein obstruction or resection [52–55]. This condition may lead to a porto-caval collateral circulation with variceal vessel transformations, especially in the lower esophagus and the cardia. As the coronary vein is a vessel of a rather large diameter, this implies that it has the capacity to drain both the spleen and the stomach, which lowers the risk of the thrombotic occlusion of the coronary-portal vein anastomosis on the one hand and makes the occurrence of left-sided portal hypertension unlikely, on the other hand.
8.4.2 Limitations of Venous Resections
The two major limitations in venous resections are: (1) a tumor infiltration which is located in a peripheral position of the SMV and does not allow to prepare a sufficient diameter of the distal vessel for an adequate anastomosis and (2) a cavernous transformation of the venous system in terms of SMV/PV thrombosis and consecutive collateral perfusion (Fig. 8.5).
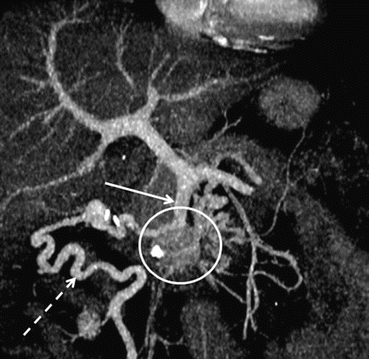

Fig. 8.5
Limitation of portal vein resection. Complete occlusion of the distal SMV by a large PDAC (white circle). Collateral vessels and main circulation via the ileocolic vein (broken white arrow) to the proximal SMV (white arrow)
The first situation must generally be regarded as a contraindication to resection as no adequate drainage of the small bowel can be achieved or it is associated with a very high risk of postoperative thrombosis of the reconstruction. Neoadjuvant therapy is not useful as it will not lead to a revascularization of the SMV and therefore will not result in resectability.
With regard to venous thrombosis and collateralization, two aspects need to be critically evaluated. On one hand, a resection may solve the problem as the collaterals—which are removed during pancreatic head resection—are replaced by the anastomosis with a free venous drainage of the intestine. Prerequisite for this approach are sufficient diameters of the PV towards the liver hilum and the SMV towards the small bowel. On the other hand, interventional approaches with preoperative stent placement to overcome thrombosis with a consequent decrease of the collateralization have been reported [56]. These attempts include endovascular stenting of the PV/SMV axis which can facilitate resection technically and reduce intraoperative blood loss as the collateral vessels can be controlled [56].
8.5 Outcome of Venous Resection
Venous resections on PDAC surgery can be performed safely, which has been demonstrated in large series that showed surgical morbidity and mortality rates comparable to pancreatic head resections without vascular involvement [25, 26, 43]. Consequently, the recommendations to perform venous resections to achieve a radical (R0) tumor removal have been implemented in national and international guidelines as well as consensus statements [4, 5, 57]. These recommendations are meanwhile scientifically examined and based not only on large cohort studies but on systematic reviews and meta-analyses [8, 9].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree