Figure. 15.1
Exposure of the infrapancreatic superior mesenteric vein (SMV). The lesser sac is opened widely by elevating the omentum off the transverse colon and reflecting it cephalad with the stomach. The hepatic flexure is then mobilized inferior and towards the patient’s left to expose the head of the pancreas and SMV inferior to the pancreatic neck
15.2.2 Step 2: Extended Kocher Maneuver
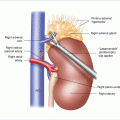
The Kocher maneuver is begun at the third part of the duodenum by identifying the inferior vena cava. All tissue medial to the right gonadal vein and anterior to the inferior vena cava is elevated, along with the pancreatic head and duodenum as the dissection progresses in a cephalad direction. This dissection is continued medially to the left lateral edge of the aorta, with exposure of the anterior surface of the left renal vein (Fig. 15.2). A complete Kocher maneuver is necessary for the subsequent dissection of the pancreatic head from the SMA (Step 6). Particularly important is the division of the leaf of peritoneum that extends from the retroperitoneum onto the root of the small bowel mesentery; incision of this portion of peritoneum is perhaps the most important part of the Kocher maneuver and allows one to appreciate the origin of the SMA.
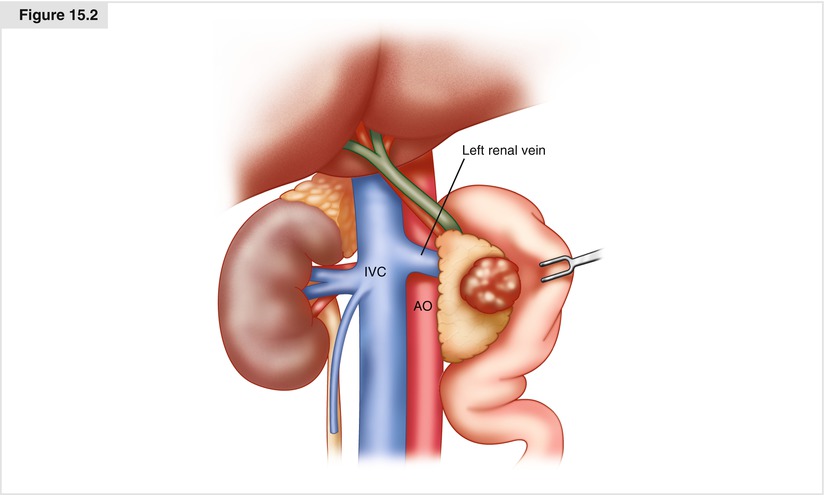

Figure. 15.2
An extended Kocher maneuver. All tissue anterior to the IVC and leftwards of the right gonadal vein is elevated along with the duodenum and pancreatic head and dissection carried out to the left of the aorta, thereby exposing the left renal vein. IVC inferior vena cava, Ao aorta
15.2.3 Step 3: Dissection of the Porta Hepatis, Hepatic Artery Exposure, Cholecystectomy, and Bile Duct Transection
The portal dissection is commenced by exposing the common hepatic artery (CHA) proximal to the right gastric artery and the gastroduodenal artery (GDA); this exposure is facilitated by removing the nodal tissue anterior to this vessel. Proximal identification of the CHA is critically important to help prevent injury to a redundant hepatic artery. Both the right gastric artery and the GDA are then ligated and divided (Fig. 15.3). Division of the GDA allows mobilization of the hepatic (common-proper) artery off of the underlying portal vein (PV), which is widely exposed along its anterior surface. Cholecystectomy is then performed, and the common hepatic duct is transected at or above its junction with the cystic duct. Following transection of the bile duct, bile cultures are sent if the bile is contaminated from the presence of an indwelling endobiliary stent. A bulldog clamp is often placed on the transected hepatic duct to prevent contaminated bile from soiling the right upper quadrant until biliary reconstruction is completed. Note that the PV should always be exposed and the location of the right hepatic artery noted prior to dividing the common hepatic duct.
Though the PV should be identified, it is not extensively mobilized until Step 6, at which time the stomach and pancreas have been divided. Care must be taken to avoid injury to the superior pancreaticoduodenal vein draining the pancreatic head at the superolateral aspect of the PV, in order to prevent significant bleeding when adequate exposure and vascular control have not yet been achieved.
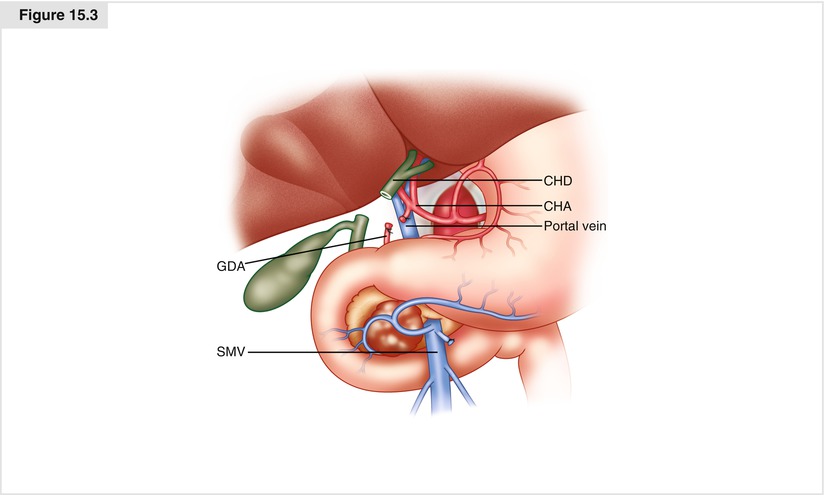

Figure. 15.3
Portal dissection, cholecystectomy, and bile duct transection. The common hepatic artery is identified proximally followed by the right gastric, gastroduodenal, proper and right and left hepatic arteries. The right gastric and gastroduodenal arteries are then ligated and divided. The gallbladder is removed. The common hepatic duct is then divided after first verifying the location of the portal vein and right hepatic artery
15.2.4 Step 4: Transection of the Gastric Antrum (or the Duodenum, if Pylorus Preservation Is Planned)
The terminal branches of the left gastric artery are ligated and divided along the lesser curvature of the stomach prior to gastric transection. The gastroepiploic artery along the greater curvature of the stomach is individually ligated and divided. The stomach is then transected with a linear gastrointestinal (GIA) stapler at the level of the third or fourth transverse vein on the lesser curvature and at the confluence of the gastroepiploic veins on the greater curvature to complete a standard antrectomy (Fig. 15.4). The omentum is then divided at the level of the greater curvature transection.
In patients with small tumors that do not invade the pyloric region or are not accompanied by metastatic adenopathy in the peripyloric region, preservation of the pylorus may be considered. It should not be performed in patients with bulky tumors of the pancreatic head, neoplasms involving the first or second portions of the duodenum, or lesions associated with grossly positive pyloric or peripyloric lymph nodes. When pylorus preservation is performed, we divide the gastroepiploic arcade and the duodenum at least 2 cm beyond the pylorus, if possible. At the time of reconstruction, we trim a few more millimeters off of the duodenum to create the duodenojejunostomy 1–2 cm from the pylorus, to ensure an adequate blood supply to the duodenum.
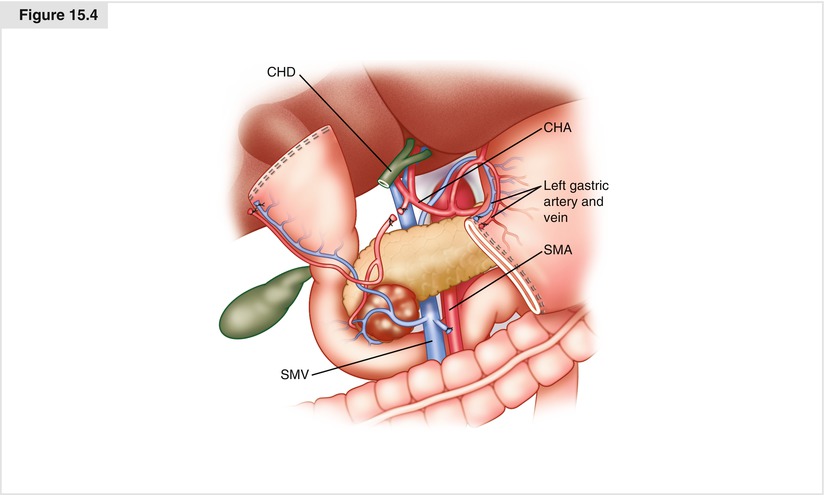

Figure. 15.4
Transection of the gastric antrum. The terminal branches of the left gastric artery are ligated and divided along the lesser curvature of the stomach, the gastroepiploic artery along the greater curvature of the stomach is individually ligated and divided, and the stomach is then transected with a linear gastrointestinal (GIA) stapler at the level of the third or fourth transverse vein on the lesser curvature and at the confluence of the gastroepiploic veins on the greater curvature, completing a standard antrectomy. CHA common hepatic artery, CHD common hepatic duct, SMA superior mesenteric artery, SMV superior mesenteric vein.
15.2.5 Step 5: Transection of the Jejunum
The loose attachments of the ligament of Treitz are taken down with care to avoid injury to the inferior mesenteric vein (IMV) situated immediately to the patient’s left, running caudal to cranial. The jejunum is then transected with a linear GIA stapler approximately 8–10 cm distal to the ligament of Treitz (Fig. 15.5), and its mesentery is sequentially ligated and divided with an energy device such as the LigaSure™ (Covidien, Mansfield, MA). This dissection is continued proximally to involve the fourth and third portions of the duodenum. The duodenal mesentery is divided to approximately the level of the aorta, allowing the devascularized segment of duodenum and jejunum to be reflected beneath the mesenteric vessels into the right upper quadrant.
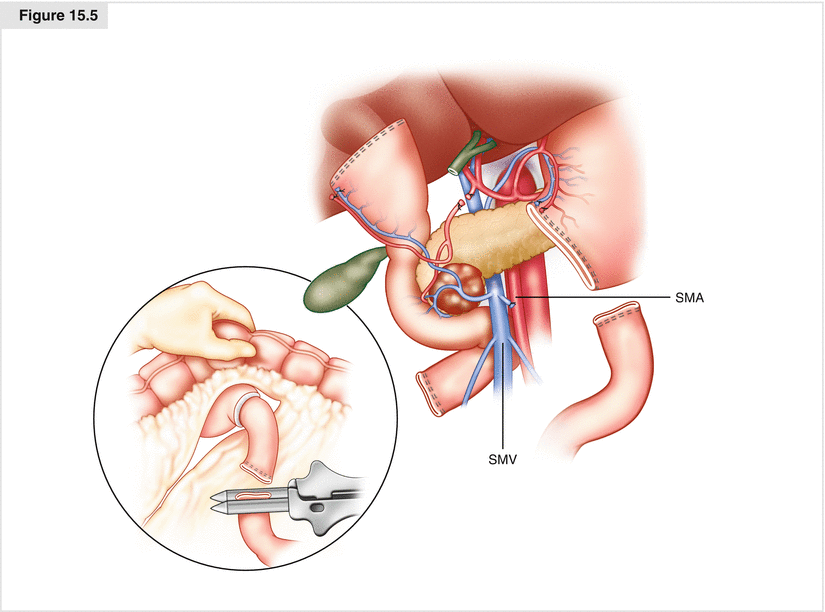

Figure. 15.5
Transection of the jejunum. The attachments of the ligament of Treitz are taken down and the jejunum is then transected with a linear GIA stapler 8–10 cm distal to the ligament of Treitz. The mesentery is sequentially ligated and divided allowing the devascularized segment of duodenum and jejunum to be reflected beneath the mesenteric root into the right upper quadrant. SMA superior mesenteric artery, SMV superior mesenteric vein
15.2.6 Step 6: Transection of the Pancreas and Completion of the Retroperitoneal Dissection
The most important and difficult part of the operation involves complete mobilization of the SMV/PV confluence and separation of the specimen from the right lateral border of the SMA (SMA margin). We usually place traction/hemostatic sutures on the superior and inferior borders of the neck of the pancreas. This is especially important along the inferior border of the pancreas, where a small artery will be found in most patients. The pancreas is divided at the level of the pancreatic neck anterior to the SMV/PV confluence. We usually use cautery to divide the pancreas and we are very careful to aspirate the pancreatic fluid when entering the pancreatic duct. The pancreatic duct is most commonly posterior in location within the pancreas and lies just a few millimeters anterior to the SMV/PV. We prefer not to try to develop a plane of dissection anterior to the SMV/PV and posterior to the pancreatic neck prior to pancreatic transection, as bleeding prior to pancreatic transection can be difficult to control and complicates (unnecessarily) this part of the operation. If the tumor encases or is adherent to the SMV/PV, the pancreas is divided to the patient’s left of the pancreatic neck in preparation for segmental venous resection. This is more difficult as the distal splenic vein is easily injured during pancreatic transection. Being very careful to locate the distal splenic vein before and during pancreatic transection (combined with experience) will minimize this complication.
The specimen is then separated from the PV and SMV by ligation and division of the small venous tributaries to the uncinate process and pancreatic head (Fig. 15.6a). Note that the relationship of the tumor to the lateral and posterior walls of the SMV/PV confluence can be directly inspected only after gastric and pancreatic transection. This relationship cannot be accurately assessed intraoperatively by simply developing a plane of dissection between the anterior surface of the SMV/PV confluence and the posterior aspect of the neck of the pancreas—which is why this age-old maneuver was abandoned.
Complete separation of the uncinate process from the SMV and its first jejunal branch is required to mobilize the SMV/PV confluence and reflect it to the patient’s left. This is necessary for proper identification of the SMA unless the SMA has been exposed medial to the SMV (which we do in the setting of very large tumors and when the root of the small bowel mesentery must be resected with the pancreatic tumor). The first jejunal branch of the SMV originates from the right posterolateral aspect of the SMV at the level of the uncinate process, usually travels posterior to the SMA, and enters the medial (proximal) aspect of the jejunal mesentery, giving off two or three branches directly to the uncinate process that need to be divided (Fig. 15.6b). If tumor involvement of the SMV at this location prevents dissection of the uncinate process from the SMV, the first jejunal branch can be divided. Injury to the SMV at the level of the first jejunal branch, or a tangential laceration in the first jejunal branch (as it courses posterior to the SMA), is hard to control and represents the most frequent cause of iatrogenic SMA injury (during efforts to control the venous hemorrhage). Whenever necessary (and certainly if the jejunal branch requires transection), we first expose the SMA medial to the SMV before dissecting the junction of the SMV and its first jejunal branch. Exposure of the SMA medial to the SMV is an underutilized technique that will always prevent an iatrogenic arterial injury or an unnecessary injury to the first-order branches of the SMV.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree