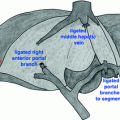
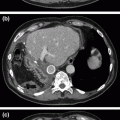
Fig. 34.1
a A residual pseudocyst is evident on repeat CT with indwelling percutaneous drain in place. Note compression of the posterior wall of the stomach. b Drainage of thin fluid and pus from the pseudocyst cavity upon placement of the self-expanding metal stent prior to balloon dilation and placement of an additional double-pigtail plastic stent. c Near-resolution of the pseudocyst, with the dumbbell-shaped metal stent in good position and plastic stent traversing through it
Because the residual cystic cavity appeared be easily accessible through the posterior wall of the stomach and continued to demonstrate compression of the gastric wall, he was determined to be an excellent candidate for endoscopic drainage. Upper endoscopy revealed extrinsic compression of the posterior gastric body. Endoscopic ultrasound identified a 6.8 × 5.0 cm fluid collection in this region containing fluid and only a small amount of solid debris. Thus, while the cystic collection (which followed an episode of necrotizing pancreatitis) might more properly be termed WOPN, its predominantly fluid-filled nature more closely resembled a pseudocyst for practical purposes. There was no obvious evidence of communication with the main pancreatic duct, but the persistent high-output fistulous drainage suggested that this was likely the case. Using color Doppler imaging to identify any interposed vessels between the walls of the stomach and pseudocyst, the cyst was punctured under endosonographic guidance and a wire was inserted under fluoroscopic guidance. Thin fluid and thicker purulent material drained from the cyst (Fig. 34.1b); the fluid was sent for amylase level which later proved to be greater than 20,000 U/L. A larger cystotomy was made with an over-the-wire cautery-enhanced lumen-apposing self-expandable metal stent. The cystotomy was dilated to 12 mm using a balloon dilator, and a 10-French double-pigtail plastic stent was placed across the metal stent to prevent intermitted occlusion by necrotic debris. While in the recovery area following the procedure, he briefly developed a fever to 38.7 °C and was started on broad-spectrum antibiotics while awaiting culture results from the pancreatic fluid. This eventually grew Streptococcus constellatus and coagulase-negative Staphylococcus, and he was transitioned to oral ciprofloxacin and clindamycin for a total of 7 days.
He tolerated sips of water on the day of the procedure and was advanced to a general diet within 2 days. His symptoms of left upper quadrant pain and early satiety resolved, his percutaneous drain was clamped, at its output had decreased dramatically, and he was discharged home on post-procedure day three. At that time, repeat CT showed that his pancreatic fluid collection had nearly resolved, with only a 1.5 × 1.2 cm residual collection remaining (Fig. 34.1c). He passed the plastic stent with a bowel movement at 2 weeks following the procedure, with no subsequent development of abdominal complaints. Surveillance MRI/MRCP at 5 weeks post-procedure demonstrated resolution of the collection. He continues to be free of symptoms from the pseudocyst at 2 months of follow-up; he has gained 10 lb, and his metal stent and percutaneous drain were removed at 3 months.
Case 2
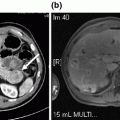
A 52-year-old man with ulcerative colitis initially presented to pancreatology clinic after being referred for refractory back pain following an episode of severe acute necrotizing pancreatitis 11 months earlier that had been attributed to alcohol abuse. He did well after his initial 3-week admission, but 8 months later developed constant pressure-like back pain that was accompanied by abdominal cramping and occasional nausea with vomiting. His pain was poorly controlled despite multiple pain-control regimens. He also reported weight loss and steatorrhea consistent with exocrine insufficiency that improved with pancreatic enzyme replacement. His type 3c diabetes was relatively well controlled with long- and short-acting insulin supplementation. His lipase, triglyceride, and IgG4 levels were within the normal ranges. MRI/MRCP obtained prior to his presentation revealed a 13.5 × 5.8 cm well-encapsulated fluid collection nearly completely replacing the pancreatic parenchyma, associated with narrowing of the common bile duct and the confluence of the superior mesenteric and splenic veins (Fig. 34.2a). Subsequent pancreas-protocol CT confirmed the appearance of a large pancreatic pseudocyst with similar measurements and findings consistent with compression of adjacent structures (Fig. 34.2b).
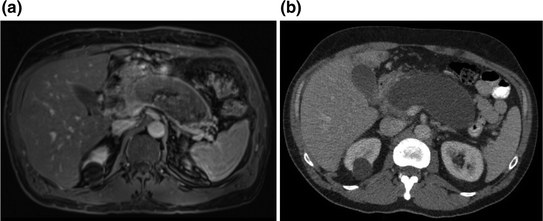

Fig. 34.2
a MRI with evidence of pancreatic parenchyma replacement by the pseudocyst, and essentially complete obliteration of the SMV-splenic vein confluence. b Pancreas-protocol CT with evidence of compression of adjacent structures, and poor apposition with the stomach and distal portions of the duodenum. Also note abutment of the splenic vein posteriorly, and several loops of jejunum and colon surrounding the lateral and anterior borders of the pseudocyst
The gastric body and splenic vein were noted to be displaced somewhat superiorly, the gastric antrum and transverse colon were veiled over the anterior aspect of the pseudocyst, and the lateral and posterior borders of the pseudocyst were closely associated with the spleen, left kidney, and loops of mid-jejunum. Because of these anatomic relationships, endoscopic and percutaneous approaches for drainage were felt to be unsafe. He was therefore evaluated in our pancreatic surgery clinic and underwent operation the following week. Diagnostic laparoscopy confirmed only limited apposition of the posterior gastric wall to the pseudocyst, a finding that was felt to preclude safe laparoscopic transgastric decompression. Instead, the procedure was converted to laparotomy and, after localization and aspiration of murky fluid from the pseudocyst, a 3-cm hand-sewn cystgastrostomy was created with a double-armed running locked Connell suture using absorbable monofilament. The pseudocyst cavity was almost entirely fluid-filled and contained only minimal solid necrotic debris. The patient tolerated a diabetic diet by postoperative day two and was discharged the following day on oral narcotic pain medication. He continued to have full symptomatic resolution over nearly 3 years of follow-up.
Case 3
A 54-year-old man who was initially diagnosed with recurrent pancreatitis of unknown etiology 16 months earlier presented to gastroenterology clinic with persistent vague upper abdominal pain associated with weight loss and decreased appetite. At presentation, lipase, triglyceride, and IgG4 levels were normal, and genetic panel testing that had been performed at an outside facility was negative for autoimmune or genetic/hereditary causes of pancreatitis. Abdominal ultrasound obtained at an outside center revealed a cystic mass in the left upper quadrant that was incompletely visualized. Subsequent MRI/MRCP demonstrated a 14.3 × 8.1 cm cystic mass in the distal pancreas with total replacement of the pancreatic body and tail that was felt to represent a pseudocyst, although a cystic neoplasm could not be excluded (Fig. 34.3a). The portal confluence was severely compressed with splenic thrombosis and associated collateral vessels. Endoscopic ultrasound was performed for further characterization, and again identified a septated, thin-walled cystic mass within the distal pancreas (Fig. 34.3b) as well as prominent adjacent vessels consistent with gastric varices. Because there was limited contact between the posterior gastric and cyst walls, and because neoplasm could not be excluded, fine-needle aspiration was performed for laboratory analysis, which revealed thin fluid with an amylase level of 9830 U/L, low CEA, and non-malignant cells on cytologic evaluation. Following this procedure, he developed mild recurrent pancreatitis with persistent fever that was treated with oral antibiotics and allowed to resolve prior to further invasive therapy.
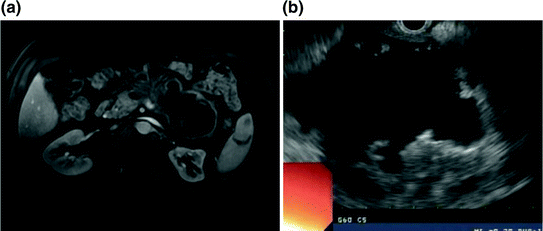

Fig. 34.3




a MRI with evidence of a large, thin-walled, septated cystic pancreatic mass. Note loops of jejunum and colon draped over the anterior surface of the pseudocyst, with no close apposition to the stomach, which precluded safe endoscopic management. b Endosonographic appearance of the septated cystic mass without direct apposition between the stomach and cyst wall
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree