Grade
A
B
C
Clinical condition
Well
Often well
Ill appearing/bad
Specific treatmenta
No
Yes/no
Yes
US/CT (if obtained)
Negative
Negative/positive
Positive
Persistent drainageb (after 3 weeks)
No
Usually yes
Yes
Reoperation
No
No
Yes
Death related to POPF
No
No
Possibly yes
Signs of infections
No
No
Yes
Sepsis
No
Yes/No
Yes/No
Readmission
No
Yes/No
Yes/No
Of note, the POPF grade can be assessed only retrospectively after the complete resolution of the clinical condition. This reflects the aim of ISGPF classification to provide a reliable definition and grading of POPF, without an actual prognostic value while the condition is ongoing.
30.2 Clinical Presentation
POPF is defined by an initial amylase-rich effluent, as well as the presence of clinical signs and symptoms or radiological evidence of peripancreatic fluid collections [5, 6]. Patients with clinically relevant fistulas (both B and C grades) usually show specific clinical manifestations since the first postoperative days. However, in a minority of cases, symptoms can be non-specific and leading to a late identification of the fistula. Non-specific symptoms can be abdominal pain, nausea, vomiting, and failure to pass flatus or stool. Clinical manifestations include fever greater than 38 °C; postural hypotension; a tender, distended, or rigid abdomen; localized abdominal or wound erythema; and warmth or swelling [1, 5, 6]. Both the quality and the quantity of drain emission are important diagnostic parameters. When a fistula occurs, the effluent is usually defined as “sinister” [1], and its appearance depends on the pancreatic juice activity on the surrounding tissues and on the presence of an anastomotic dehiscence. Quality can be classified as “pure” when the effluent constituted exclusively of pancreatic juice or “mixed” when pancreatic juice is combined with bile or enteric fluid [5]. Many other definitions are present in the literature including “Coca-Cola-like,” “murky,” “whitish,” “bilious,” “bloody,” “purulent,” and “foul smelling” when a infection occurs [4]. Moreover, POPF can be classified as of “low” or “high output,” depending on whether the daily volume of fluid exceeds the 200 ml. Based on the timing of manifestation, POPF can be defined as “early” if it occurs within the first week after surgery or “latent” when an initial low drain amylase activity occurs, but ultimately the patient exhibits the clinical or radiological findings of a POPF (about 5% of all resections) [5, 6]. In contrast with an early POPF, latent is usually more severe and twice as likely to be infected. In these cases hospital readmission is required, and the duration of the staying is significantly longer and associated with higher costs [6].
30.3 Risk Factors
The POPF development is related to a multifactorial condition, where the major role is played by patients, pathological, and surgical factors [7]. The most significant risk factors for the development of POPF after pancreaticoduodenectomy (PD) are a soft pancreatic parenchyma and a non-dilated main pancreatic duct [8, 9], as both affect the reconstruction of the pancreatic-enteric anastomosis. It is well established that a “healthy” pancreatic parenchyma without fibrotic modifications is more susceptible to injury during the operative dissection and the anastomosis confectioning. In this case, the texture is friable, and sutures are more vulnerable to tear through the parenchyma as well as through a fragile duct lining. Moreover, a small pancreatic duct is more challenging to be reconstructed, with a significant probability to occlude or dehisce once the anastomosis is performed. The pathophysiological mechanism behind the POPF development is related to the exocrine function that is generally preserved in soft pancreatic glands, resulting in a preserved secretion of the pancreatic juice rich in proteolytic enzymes. Neoplasms that infiltrate the main pancreatic duct determining upstream chronic pancreatitis, such as adenocarcinoma, are often associated with a harder parenchyma and a dilated main pancreatic duct (MPD). Conversely, masses that do not occlude the MPD like cystic, neuroendocrine, and ampullary tumors are considered as risk factors for POPF.
Other factors are associated with POPF and include age, comorbidities, body mass index, jaundice, neoadjuvant chemotherapy, pancreatic steatosis, intraoperative blood loss, and operative technique [7]. Based on these factors, several predictive scores have been developed to stratify the patient’s risk to develop POPF after PD [7, 8, 10–12]. In particular, Callery et al. [8] proposed a risk score that considers the preoperative diagnosis and other intraoperative data such as the gland texture, the pancreatic duct diameter, and the intraoperative blood loss (Table 30.2). The assessment of these factors into scores has been used to assign each patient into a risk zone: negligible (0 points), low (1–2 points), moderate (3–6 points), and high (7–10 points), with a good correlation with POPF development [13]. Determining the pre- or intra-operative POPF risk allows clinicians to a proper informed consent, as well as to individualize operative and postoperative conduct. Recent evidences [14] suggest that these scores may help to recognize low-risk patients suitable for no drain placement, as well as high-risk patients in which additional treatment should be advocated. Specifically, in these patients clinicians may decide to start a prophylactic treatment with somatostatin or its analogues. In addition, they might consider other intraoperative strategies such as an upfront total pancreatectomy, to perform a pancreaticogastrostomy over than a classical pancreaticojejunostomy, the placement of intraductal stents, or a feeding jejunostomy [8, 15, 16].
Table 30.2
FRS for the prediction of CR-POPF after pancreatoduodenectomy
Risk factor | Parameter | Points |
|---|---|---|
Gland texture | Firm | 0 |
Soft | 2 | |
Pathology | Pancreatic adenocarcinoma or pancreatitis | 0 |
Ampullary, duodenal, cystic, islet cell, etc. | 1 | |
Pancreatic duct diameter | ≥5 mm | 0 |
4 mm | 1 | |
3 mm | 2 | |
2 mm | 3 | |
≤1 mm | 4 | |
Intraoperative blood loss | ≤400 ml | 0 |
401–700 ml | 1 | |
701–1000 ml | 2 | |
>1000 ml | 3 | |
Total 0–10 points |
30.4 Treatment
The early treatment of POPF is conservative. This consists in limiting the oral intake, administering somatostatin analogues to inhibit the pancreatic exocrine secretion, and providing adequate nutritional support and, if necessary, antibiotics. If this first-line management fails and imaging demonstrates non-drained abdominal collections, interventional procedure might be warranted. Finally, a reoperation may be required in selected critical patients whenever minimally invasive approaches fail to improve the clinical condition.
30.4.1 Somatostatin and Its Analogues
As previously stated, the active exocrine secretion of pancreatic enzymes has a key role in the development of POPF. During the last decade, several pharmacological approaches have been investigated in order to successfully inhibit the pancreatic exocrine secretion. Somatostatin is a 14-amino acid peptide that has an active role on the digestive system by inhibiting pancreatic exocrine, biliary, and small bowel secretions and increasing the water absorption [18]. When any digestive fistula occurs, somatostatin reduces its output with potential positive effects on its natural course. The major limitation of somatostatin is its very short half-life (1–2 min), necessitating for continuous intravenous infusions. In order to avoid long infusions, synthetic analogues such as octreotide and pasireotide are nowadays available (with a half-life of 120 min and 11 h, respectively) [18, 19]. These analogues allow for intermittent subcutaneous dosing schedules and differ from each other in the binding profile for somatostatin receptors [18, 19].
Several randomized controlled trials were conducted with the aim to demonstrate the effectiveness of somatostatin and its analogues on POPF mitigation, but results are conflicting [20, 21]. The main bias of these trials was substantially represented by the heterogeneity with respect to the definition of POPF, the type of analogues administered, the pancreatic resection performed, and the timing of the treatment. In 2013, a Cochrane systematic review [21] compared the use of somatostatin analogues with a no-somatostatin group in pancreatic surgery. Authors reported no differences in the incidence of clinically significant POPF and in the length of staying, but with lower overall postoperative complications in the interventional group. Authors concluded that considering the lack of serious adverse effects and the relatively low costs, somatostatin analogues should be recommended routinely in pancreatic surgery [21]. Recently, another randomized controlled trial considering only high-risk patients failed to demonstrate the aforementioned benefits for the octreotide group [22]. Interesting results have been reported by Allen et al. [19] in a randomized, double-blind placebo-controlled trial on pasireotide. Authors demonstrated a significant reduction of clinically relevant POPF in the pasireotide group (7.9% vs 16.9%) with no grade C fistula occurring in this cohort [19]. After over 20 years and many studies, there are still no definitive conclusions on this matter. For these reasons, further high-quality RCTs are necessary before considering somatostatin and its analogue as standard treatment in pancreatic surgery.
30.4.2 Parental and Enteral Nutrition
Nutritional support is an essential element in the management of patients with clinically relevant POPF, either through parental nutrition (PN) or enteral nutrition (EN). Most of these patients need to be kept with “nothing by mouth” as the oral food intake enhances the pancreatic juice secretion [1]. In addition, artificial nutrition could improve the wound healing necessary for the anastomotic leak closure [23]. Postoperative malnutrition may be associated with high-output POPF (>200 mL of daily exocrine secretion), leading to a significant loss of fluid and electrolyte imbalance [24]. In this matter, EN is considered by the last evidences as the nutritional support of choice, avoiding the well-known disadvantages related to long-term PN such as infections (septicemia, wound infections) and metabolic complications (hyperglycemia) [24–27]. EN prevents the atrophy of gastrointestinal mucosa and preserves the intestinal bacterial flora architecture, resulting in the inhibition of the microbial translocation from the gut to the bloodstream [23, 28]. In addition, an open-label randomized controlled clinical trial by Klek et al. [27] demonstrated that EN, compared to PN, is associated with an increased faster rate of POPF closure and with a lower rate of nutrition-related complications.
In case of clinically relevant POPF, EN is administered via a nasal-jejunal tube or a jejunostomy tube. The rationale consists in a continuous feeding of the distal jejunum, allowing for a resting in the exocrine pancreatic secretion [29]. For this reason, the tube must be placed distal to the pancreas, below the Treitz’s ligament or, in case of a PD, distal to entero-enteric anastomosis [17, 29].
Of note, the EN therapy for POPF needs to be adjusted according to the course of the complication and the intestinal tolerance. The ESPEN guidelines suggest an energy supply that should not exceed 20–25 kcal/kg BW/day during the acute and initial phase of critical illness, which can be increased up to 25–30 kcal/kg BW/day during the anabolic recovery phase. If these target values are not reached, supplementary PN should be added [30]. In elective upper gastrointestinal surgical patients, EN with immune-modulating formulae (enriched with arginine, nucleotides, and omega-3 fatty acids) is superior to standard enteral formulae [30]. Currently there is no agreement on routine use of tube feeding in pancreatic surgery [31, 32], but an intraoperatively prophylactic placement should be at least considered in patients with high risk to develop POPF [8].
30.4.3 Interventional Radiology and Endoscopic Therapy
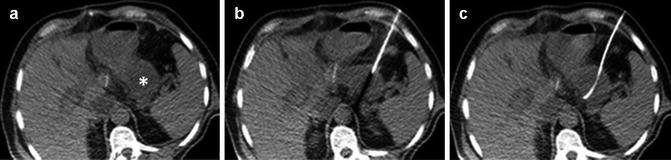
The role of interventional radiology in the POPF treatment is becoming increasingly relevant. Currently, around 7–12% [33, 34] of pancreatic resections are complicated by abdominal fluid collections, and these are mostly due to POPF that are not properly drained. These collections are rich in amylase and may lead to potentially fatal complications. Patients might be initially asymptomatic or suffer from mild abdominal discomfort and pain. Subsequently, the typical signs of infections might develop despite the antibiotic therapy, such as fever and leukocytosis, eventually leading to sepsis [35]. In the most serious cases, if the patient is hemodynamically stable, imaging-guided percutaneous drainage may offer a safe alternative to surgical exploration, as it is less invasive, requires shorter recovery time, and is associated overall to a lower morbidity [36]. In recent series, the 5–7% of PD and up to 14–20% of distal pancreatectomy (DP) [34, 37–39] developed intra-abdominal collections requiring a percutaneous drainage. This procedure might be either ultrasound (Fig. 30.1) or computed tomography guided, according to the operator expertise, and mostly using the Seldinger or the tandem-trocar technique [36, 37, 40]. The aspirated fluid must be sent for amylase value assessment and microbiology cultures. The catheter should be as large as possible since the collected fluid is often viscous and hard to drain. The cause of this viscosity might be related to the presence of pus and bile or to the local fat necrosis due to the pancreatic juice leak [37]. Imaging-guided percutaneous drainage is associated with high technical success rates (95–100%) [37, 40, 41]. However, more than 30% of patients require a second procedure (catheter exchange, increase in catheter size, catheter repositioning, additional catheter) [37]. The related morbidity is low but not negligible, and it includes bleeding and visceral perforation [36, 40, 41]. Recently, endoscopic ultrasound (EUS)-guided drainage has become a viable alternative for the treatment of peripancreatic fluid collections. EUS drainage avoids the implications of external drainage such as the frequent need for maintenance, the risk of local skin irritation and infection, and the electrolyte loss, resulting in an improving of quality of life. The procedure is performed under sedation or general anesthesia, using standard endoscopic supplies. The drainage is achieved by passing through the gastric or the duodenal wall and controlled, thanks to the high-resolution and real-time imaging of the pancreas and the surrounding vasculature. The tract is then safely dilated, and one or more double pigtail stents are left in place, allowing for a rapid fluid evacuation [41, 42]. Although the experience is still limited, the reported technical success rate is around 100% with a clinical success rate of 93–97%, even though one out of three patients would require a second EUS drainage [41, 42]. Most of these experiences refer to patients who underwent DP [41, 42] although series including more challenging PD cases exist [41, 43]. The interventional timing could be another limiting factor for EUS-guided drainages, as a thicker wall surrounding the collection is needed. However, while most of the studies excluded patients with fluid collection less than 4 weeks old because of the presumed lack of the collection wall [40, 42], Tilara et al. [35] recently reported a series of 17 patients who underwent early drainage (<30 postoperative days) showing that EUS-guided drainage is feasible and safe.