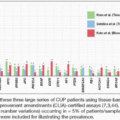
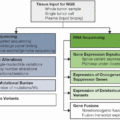
Pancreatic cancer is the third most common cancer causing mortality in the United States. Despite improvements in mortality rates observed in many other cancer types in the last 5 to 10 years, the mortality rate from pancreatic cancer has been stable in women and increasing in men at 0.3% per year since 2000 (
1). The majority of patients (52%) who are diagnosed with pancreatic cancer have distant metastatic disease at diagnosis (
1), and even in patients with resectable disease who are treated with adjuvant chemotherapy, 3-year disease-free survival rate is only 39.7% with adjuvant FOLFIRINOX chemotherapy (
2) and 20.9% with adjuvant gemcitabine + capecitabine (
3), demonstrating the high risk of recurrence even in patients with earlier stage disease. For patients with metastatic disease, the standard of care first-line chemotherapy regimens include FOLFIRINOX and gemcitabine + nab-paclitaxel, but median overall survival is only 8.5 to 11.1 months (
4,
5). Until recently, there were no predictive biomarkers to enable selection of patients to receive targeted therapy or immunotherapy. However, with emerging data, national guidelines now recommend testing pancreatic cancer for germline mutations, somatic mutations and fusions, and microsatellite instability/mismatch repair deficiency (
6), as results impact both standard of care options and eligibility for promising clinical trials of targeted therapies with compelling preclinical data.