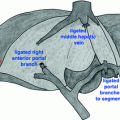
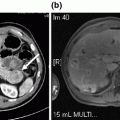
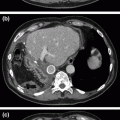
Fig. 23.1
Diagnostic imaging with contrast-enhanced computed tomography (CT) scan. Our diagnostic preference is to obtain a high-quality, intravenous contrast-enhanced CT scan. The scan should be completed with dual phase images, once during arterial enhancement and once during portal venous enhancement. Here we show our patient with a 5 cm mass in the neck/body of the pancreas (white arrow) that completely encases the celiac axis and its proximal branches
Diagnosis and Staging
A diagnosis of PDA can be made definitely based on histopathologic analysis of biopsy specimens. Typical findings include pleomorphic and hypercellular fragments of tissue with ductal features in a relative paucity of acinar epithelium. The nuclei are characteristically enlarged with irregular contours. Multinucleated cells and mitosis are often encountered. Cells are often found to be arranged haphazardly and with a lack of discernible polarity, often described as a “drunken honeycomb” arrangement [6]. Once the diagnosis has been confirmed, information gleaned from the patient’s physical exam, imaging studies, endoscopy, and histology are combined to accurately stage the patient’s disease.
The most common staging system used for PDA (Table 23.1) is derived from a consensus of experts in conjunction with the American Joint Committee on Cancer with a goal of facilitating treatment decisions and prognosis [7]. Currently in its seventh edition, the backbone relies on an evaluation of the primary tumor (T-stage), regional lymph nodes (N-stage), and presence or absence of metastasis (M-stage). Final anatomic or prognostic staging involves grouping of T, N, and M categories as can be seen in Table 23.1. The T and M-stage categories reflect the major determinant of survival in PDA: the capacity for complete surgical resection. When the primary tumor extends beyond the pancreas to involve the celiac axis or the superior mesenteric artery for greater than 180°, the term locally advanced PDA is used. This is generally regarded as unresectable disease (T4). In the absence of metastatic disease, a T4 tumor is considered stage III disease regardless of nodal status. When associated with evidence of metastasis a T4 tumor is considered stage IV.
Table 23.1
Pancreatic cancer staging
Primary tumor (T) | |||
Tis | Carcinoma in situ | ||
T1 | Tumor limited to the pancreas, <2 cm | ||
T2 | Tumor limited to the pancreas, >2 cm | ||
T3 | Tumor extends beyond the pancreas but without involvement of CA or SMA | ||
T4 | Tumor involves CA or SMA | ||
Regional lymph nodes (N) | |||
N0 | No regional lymph node metastasis | ||
N1 | Regional lymph node metastasis | ||
Distant metastasis (M) | |||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
Anatomic stage | |||
Stage 0 | Tis | N0 | M0 |
Stage IA | T1 | N0 | M0 |
Stage IB | T2 | N0 | M0 |
Stage IIA | T3 | N0 | M0 |
Stage IIB | T1 | N1 | M0 |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
Stage III | T4 | Any N | M0 |
Stage IV | Any T | Any N | M1 |
It is worth a moment to discuss issues that arise in delivering a diagnosis of PDA in the clinic to our patient. As one of the most commonly encountered malignancies causing death, she may have preconceived notions regarding the meaning of the diagnosis. Certainly, there is a fair amount of nihilism that is associated with PDA in the general public. Practically, it can be helpful to deliver this news, when the patient allows, in the presence of family and her loved ones, as this support system may be an important part of the overall treatment plans. It can also be helpful to ask what they may know of the disease and use this as a starting point for counsel and guidance with medical decision-making (reinforcing accurate notions and correcting knowledge gaps). Ultimately, it is important to provide your patient with accurate clinical knowledge so she and her family can formulate clear and consistent goals of care throughout her treatment course.
At the time of diagnosis, only 20% of PDA are amenable to complete surgical resection [8, 9]. Of the remaining 80%, approximately one-third will have locally advanced disease without evidence of metastasis (stage III). Due to a high incidence of perioperative complications and a high rate of disease recurrence with poor response to adjuvant therapies, patients with stage III disease have rarely been offered attempts at surgical extirpation in the past. However, improvements in peri-operative outcomes and responses to systemic chemotherapeutics have led to more aggressive approaches in a highly selected group of patients. In the case presented here, we will discuss the management of a stage III PDA with tumor in the pancreatic neck and body with involvement of the celiac trunk and common hepatic artery.
Technical Pearls
Diagnostic laparoscopy prior to laparotomy for planned curative resection can be used liberally in this patient cohort, as patients with unresectable or metastatic disease rarely require intestinal or biliary bypass.
Prior to addressing the technically challenging hepatoduodenal ligament and superior pancreatic dissection, assessment of the retroperitoneal vasculature can be completed by mobilizing the duodenum and right colon with wide kocherization and a Cattell–Braasch maneuver.
Liver perfusion is dependent upon retrograde flow through the gastroduodenal artery (GDA) to the proper hepatic artery. Double-check adequacy of flow with both palpation and Doppler before common hepatic artery transection. Protect the GDA by transecting the pancreas well away from its course over the pancreatic head and neck.
Preoperative Management
In a simplified model, patients such as ours with locally advanced PDA arising in the neck and body of the pancreas can be separated into two groups (Fig. 23.2). In the first group, patients are thought to have “poor” disease biology, with rapid local tumor growth and evidence of early disease metastasis. The second group is said to have “favorable” disease biology, with a long period of time during which the disease burden will remain relatively stable, without further local growth or evidence of metastasis. It is this second group that may benefit from more aggressive surgical therapies for local disease control. The rationale here is twofold: first, identification of a group of patients in whom disease is truly limited to the gland and surrounding structures where resection would therefore achieve cure (i.e., resect all disease prior to metastasis); and second, identification of a group of patients in whom survival will be driven primarily by local, rather than systemic, disease (i.e., systemic disease appears to be well controlled by chemotherapeutics).
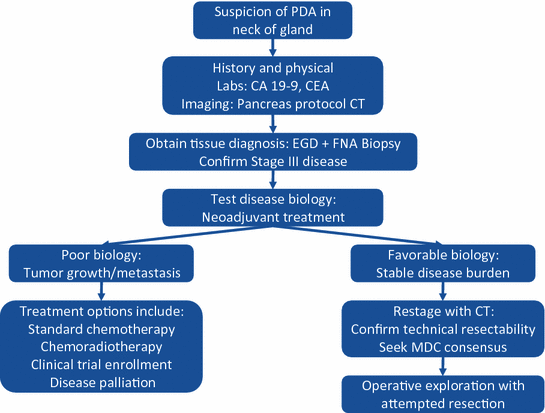

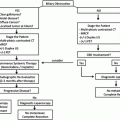
Fig. 23.2
Treatment diagram outlining the steps taken when pancreatic ductal adenocarcinoma is suspected in the neck of the gland. PDA pancreatic ductal adenocarcinoma; CA 19-9 carbohydrate antigen 19-9; CEA carcinoembryonic antigen; CT computed tomography; EGD esophagogastroduodenoscopy; FNA fine-needle aspiration; MDC multidisciplinary conference
Despite a plethora of research on the topic, there remains no prospective way to dichotomize patients into these two biologic groups based on the tissue sampling or clinical laboratory analysis at the time of diagnosis. As a result, our preferred method for patient selection based on disease biology is by treating first with non-surgical therapies and restaging at an interval. Experience with neoadjuvant approaches to stage III PDA is increasingly being reported in the literature [10–12]. Combination cytotoxic chemotherapeutics, particularly modified fluorouracil, leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX) with or without radiotherapy, is beginning to repeatedly demonstrate a capacity to downstage patients into a surgical paradigm of management. In data focused primarily on pancreatic head lesions, R0 resection in locally advanced disease can be achieved at rates exceeding 85% [10, 13, 14]. Experience with these regimens appears safe, with variable levels of toxicity based predominantly on the appearance of side effects from systemic chemotherapeutics.
Following neoadjuvant therapy, restaging of the patient should be performed as a surrogate indicator of disease biology. “Favorable” biology, as evident by disease stability or regression in combination with an absence of metastatic spread over time, should be a trigger to critically evaluate the patient’s imaging for technical barriers to surgical extirpation. In those patients with elevated CA19-9 levels upon presentation, a decrease in that laboratory value may also be an indicator of favorable disease biology. The importance of surgical resection for the group of patients with favorable tumor biology and technically resectable disease should not be trivialized. Surgical resection, even in a locally advanced cohort of patients, remains the only chance for cure. Direct tumor invasion into adjacent locoregional structures does not always preclude an operation. Shoup et al., for example, demonstrated that patients requiring multivisceral resections for PDA of the body or tail have improved survival over locally advanced patients who do not undergo resection. Further, the long-term survival after multivisceral resection is similar to that following standard pancreatic resection [15].
PDA involving the celiac (CA) and common hepatic (CHA) arteries represents a unique surgical challenge. En-bloc resection of these arterial structures has been avoided in the past due to increased perioperative morbidity and questionable benefit. As the relative oncologic benefit improves (better chemotherapies, favorable tumor biology), the perioperative morbidity may become acceptable for select patients. Increased morbidity, as compared to standard distal pancreatectomy, is largely due to the potential for ischemia from devascularization of the liver, stomach, and spleen. In practice, ligation of the splenic artery is required for splenectomy, and carries little to no added morbidity. Similarly, ligation of the left gastric artery should have little effect on a stomach with intact collateralization. In stark contrast, however, devascularization of the liver remains a concern, as collateralization through the GDA is required to maintain adequate perfusion.
Resection of the distal pancreas and spleen, en-bloc with the CA, was first described by Appleby in 1953 as a surgical therapy for locally advanced gastric cancer [16]. Modification of this original operation, leaving the stomach intact, for use in resection of locally advanced PDA was first proposed in Japan and has been subsequently reported with increasing frequency in the literature [11, 12, 17–19]. With expanded experience, many high-volume pancreas centers have now demonstrated the safety of the modified Appleby procedure for PDA. Multidisciplinary management is a key to appropriate patient selection in this cohort. For example, if concern exists regarding the adequacy of collateral flow to the liver, preoperative angiography with coil embolization can be considered and, if needed, safely utilized [11, 14].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree