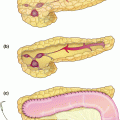
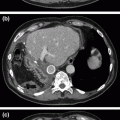
Fig. 21.1
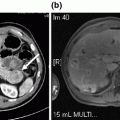
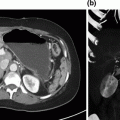
Pre-operative imaging. Axial images of venous phase pancreatic protocol CT scan demonstrating hypodense lesion in pancreatic head: a portal vein free of tumor; b abutment of the portal vein with tumor; c tumor involvement of the splenic-portal vein confluence
Management
The patient presented with borderline resectable pancreatic adenocarcinoma. He received three cycles of neoadjuvant FOLFIRINOX (folinic acid, fluorouracil [5-FU], irinotecan and oxaliplatin) chemotherapy followed by stereotactic body radiotherapy (SBRT) with a cumulative dose of 2400 cGy. There was no progression of disease during treatment and, approximately 5 weeks after his last dose of chemotherapy and 3.5 weeks after his last radiation treatment, he proceeded to pancreaticoduodenectomy (PD) with venous resection and reconstruction with interposition of the internal jugular vein and splenic vein preservation. The patient is currently alive and well, undergoing workup for possible recurrence but presently biopsy-negative, more than 2 years after PD with venous reconstruction.
The above patient case demonstrates the application of neoadjuvant therapy for pancreatic adenocarcinoma, followed by restaging and pancreaticoduodenectomy with vascular reconstruction. The first extended pancreaticoduodenectomy with concomitant superior mesenteric vein (SMV) resection and reconstruction was performed in 1951 [4].
Pancreaticoduodenectomy with portal vein (PV) resection and reconstruction as part of an en bloc resection of the pancreas and surrounding structures to improve survival was first described in Japan[5]. Nearly a decade later, a similar “regional pancreatectomy” involving resection of the major peri-pancreatic vasculature with wide soft tissue clearance was described by Fortner in the United States [6]. However, contrary to early beliefs, no survival benefit had been demonstrated for patients undergoing radical or extended PD [7, 8]. It was not until recently that venous resection (VR) has demonstrated more favorable results, with comparable survival for patients with tumor involvement of major venous structures requiring venous resection and reconstruction compared to standard PD [9].
Subsequent studies within the last decade have found that patients with locally advanced disease requiring venous resection of the superior mesenteric and/or portal veins demonstrated similar survival [10] and postoperative morbidity and mortality [11] compared to patients who underwent standard PD. Findings were similar for those limited to tumors of the head [12–14]. Also, the results were not affected when PD was performed for other indications (ampullary and distal common bile duct cancers) [15]. One study [16] and several meta-analyses [17, 18] evaluating mesenteric-portal vein resection for all pancreatectomy types found similar results for survival, mortality, and morbidity. Multiple review articles have also concluded that portal vein and/or superior mesenteric vein resection is safe and confers a presumed survival advantage [19]. One study noted that patients who underwent SMV/PV resection had decreased survival relative to patients without resection; however, this study included a heterogeneous population of resectable and borderline resectable patients [20]. Another large database retrospective review found that patients undergoing pancreaticoduodenectomy with concomitant vascular resection had significantly increased rates of perioperative mortality and morbidity compared to those who did not [21]; although the interpretation of these results has been criticized, as large database work is limited by difficulty defining entry criteria and venous resection procedures resulting in mixing of cases of emergent versus planned reconstructions, as well as the inclusion of a potentially heterogeneous patient population not described in the context of multimodality cancer treatment [22]. Only one recent large meta-analysis demonstrated that patients undergoing PV-SMV resection had increased mortality, higher rates of R1/R2 resections, and worse survival [23]. Numerous studies have found that tumor-free margins [10, 16, 23–27], as well as the presence of tumor infiltration on venous resection [16, 17, 28, 29] were the most important prognostic factors. Only one study found no survival difference in the presence or absence of venous tumor infiltration [15] (Table 21.1).
Table 21.1
Pancreaticoduodenectomy for pancreatic adenocarcinoma with venous resection reported in the literature since 2004
First author (year) | No. patients total (adeno) | No. patients (+VR) | No. patients (−VR) | % Operative mortality (+VR) | % Operative mortality (−VR) | Morbidity (+VR) | Morbidity (−VR) | Median survival (mo) (+VR) | Median survival (mo) (−VR) | No. positive margin (R1/2) (+VR) (%) | No. positive margin (R1/2) (-VR) (%) | %vein infiltrate/ specimen examined |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Tseng (2004) | 291 | 110 | 181 | 1 (0.9%) | 2 (1.1%) (p = 0.86) | 20 (18.2%) | 39 (21.5%) | 23.4 | 26.5 | 24/110 (21.8%) | 21/181 (11.6%) | 38/62 (61%) |
Poon (2004) | 50 | 12 | 38 | 0 | 1 (2.6%) | 5 (41.7%) | 16 (42.1) | 19.5 | 20.7 | 8.3% | 15.8% | 6/12 (50%) |
Riediger (2006) | 125 | 40 | 85 | 2/53* (3.8%) | 7/169* (4.1%) | 22/53* (41.5%) | 81/169* (47.9%) | 22 | 15 | 13 (33%) | 19 (24%) | 16/29 (55%) |
Carrere (2006) | 133 | 45 | 88 | 2 (4.4%) | 5 (5.7%) | 25 (55.6%) | 56 (63.6%) | 15 | 19 | 8 (17.8%) | 13 (14.8%) | 29/45 (64%) |
Ravikumar (2014) | 1070 | 230 | 840 | 10 (4.6%) | 26 (4.2%) | 151/230 (65.6%) | 432/840 (51.4%) | 18.2 | 18.0 | 144/229 (62.9%) | 423/820 (51.6%) | 150 (65.2%) # |
Cheung (2014) | 78 | 32^ | 46 | 1/32 (3.1%)+ | 2/46 (4.3%)+ | 10/32 (31.5%) | 20/46 (43.5%) | 70.6%; 33.3% *** | 71.1%; 23.6%*** | 7/32 (21.9%) | 11/45 (24.4%) | – |
Murakami (2015) | 937** | 435 | 502 | – | – | – | – | 18.5± | 25.8± | – | – | – |
Wang (2015) | 208 | 42 | 166 | 1/42 (2.4%) | 2/166 (1.2%) | 16/42 (38.1%) | 50/166 (30.0%) | 20.0 | 26.0 | 8/42 (19.0%) | 36/166 (21.7%) | 100% |
Kulemann (2015) | 338 | 131 | 208 | 2/131 (1.5%) | 8/208 (3.8%) | 73/131 (55.7%) | 104/208 (50%) | 21.6 | 19.7 | 46/131 (35.4%) | 49/208 (23.8%) | – |
Venous resection is often undertaken after neoadjuvant therapy. While neoadjuvant therapy for borderline resectable tumors has been subject to debate, the latest National Comprehensive Cancer Network (NCCN) guidelines recommend neoadjuvant therapy prior to attempted surgical resection [26, 30–32]. However, the International Study Group of Pancreatic Surgery (ISGPS) supports venous resection for borderline tumors without necessitating neoadjuvant treatment [33, 34].
Pre-operative Planning
Appropriate patient selection is crucial for successful venous resection. Radiographic imaging should be reviewed to ensure that (1) no metastatic disease is present; (2) there is no evidence of tumor involvement of the superior mesenteric artery (SMA) or celiac axis; and (3) the SMV and PV are patent without evidence of segmental or complete thrombosis [35].
The addition of vascular resection and reconstruction increases the complexity of PD and should be performed in the setting of (1) a multidisciplinary evaluation of the patient, with strong consideration of neoadjuvant treatment unless contraindicated; and (2) a high-volume surgical and perioperative team with extensive experience in vascular reconstruction and vascular surgical expertise available for preoperative and intraoperative consultation. Appropriate venous phase imaging must be obtained preoperatively to appreciate tumor abutment of the lateral or posterolateral wall of the SMV or superior mesenteric-portal vein (SMPV) confluence, the presence of which should indicate the need for venous resection [24]. Poor patient performance status and underlying organ system damage (especially hepatic or renal insufficiency) may serve as relative contraindications for vascular resection and consideration of other local therapies such as definitive SBRT may apply.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree