Fig. 13.1
Proposed progression model for pancreatic cancer. The majority of pancreatic cancer is believed to involve telomere shortening and mutations of the oncogene KRAS occurring in early stages, followed by the inactivation of the p16 tumor suppressor gene intermediately and, finally, the inactivation of the p53, SMAD4 (DPC4), and BRCA-2 tumor suppressor genes at late stages (Modified from Chang DK, Merrett ND, Biankin AV. Improving outcomes for operable pancreatic cancer: Is access to safer surgery the problem? J Gastro Hepatol 2008;23:1036–45 with permission from John Wiley & Sons, Inc.)
The proposed progression model involves telomere shortening and mutations of the oncogene KRAS that occur in the early stages, followed by the inactivation of the p16 tumor suppressor gene in the intermediate stages and, finally, the inactivation of the p53, SMAD4 (DPC4), and BRCA-2 tumor suppressor genes at late stages [8] (Fig. 13.1).
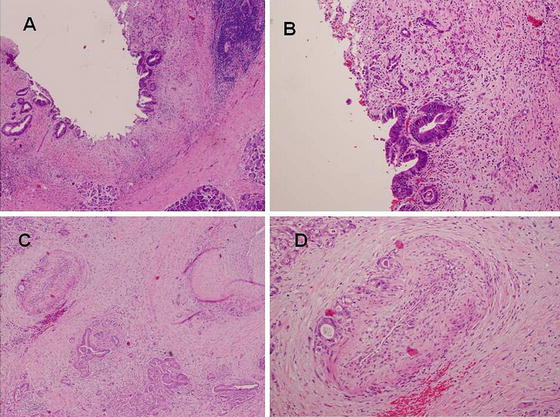
The histology of ductal adenocarcinoma is of poorly differentiated tubular structures or cell clusters; aggressive, infiltrative growth; and dense stromal fibrosis (Fig. 13.2).
Diagnosis
Signs and Symptoms
The signs and symptoms, if any, of a patient presenting with pancreatic adenocarcinoma are related to the location of the cancer within the pancreas, mainly differentiated between carcinomas of the head of the pancreas and the body or tail of the pancreas. Many patients with pancreatic head carcinomas present with weight loss and obstructive jaundice, and occasionally, there is an associated deep abdominal or back pain. Because pancreatic body-tail carcinomas are further from the bile duct, they rarely present with jaundice and may present only with weight loss and abdominal pain. Regardless of the location, the diagnosis of pancreatic cancer can be extremely difficult due to the vague presenting symptoms.
Unfortunately, no definitive early warning signs have been established [9]. The classic description of pancreatic cancer presentation is that of a patient with painless jaundice. Painless jaundice can result from obstruction at the level of the ampulla. Causes include tumors from the duodenum (i.e., duodenal tumors), bile duct (i.e., cholangiocarcinoma), or pancreas (pancreatic head tumors). Patients with pancreatic head tumors have less abdominal pain than patients with body-tail tumors. Approximately one-quarter of pancreatic cancer patients have no pain at all at the time of diagnosis [10].
One study of patients diagnosed with exocrine pancreatic cancer found that the most common presenting symptoms are fatigue, weight loss, anorexia, and abdominal pain. Jaundice, hepatomegaly, right upper quadrant mass, and cachexia are among the most common presenting signs. Changes in urine and stool are also common; choluria (dark urine) occurs in approximately 60 % of pancreatic cancer patients, and hypocholia (clay-colored stool) occurs in approximately 54 %. Additionally, Courvoisier’s sign (enlarged, non-tender, palpable gallbladder in a patient with jaundice) and migratory thrombophlebitis are also well-recognized signs associated with pancreatic cancer; these have been found in 13 % and 3 %, respectively, of patients diagnosed with pancreatic cancer [11]. Unfortunately, there is no screening test for pancreatic cancer, and as a result, most patients with pancreatic cancer have advanced disease by the time of diagnosis.
Laboratory Data
In a patient with an obstructed bile duct, elevated alkaline phosphatase and bilirubin levels are often present. Levels of the tumor marker CA19-9 are also elevated in patients with pancreatic cancer; unfortunately, this test is not sensitive enough to screen for and diagnose pancreatic cancer. It is usually used in follow-up after treatment.
Imaging Studies
Computed Tomography (CT Scan)
Computed tomography (CT scan) is a primary imaging modality and gold standard for evaluating pancreatic cancer. It is used for diagnosing primary pancreatic malignancy, assessing resectability, and evaluating for metastases. In addition, CT scans can show anatomic anomalies such as a replaced right hepatic artery, which generally originates from the superior mesenteric artery (SMA) in 25 % of patients (Fig. 13.3).
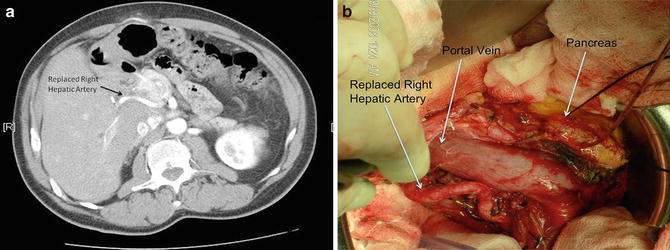

Fig. 13.3
CT scan demonstrated a replaced right hepatic artery originating from the superior mesentery artery (a) and an intraoperative picture of the same patient with a replaced right hepatic artery (b). Note that this arterial anomaly can occur in 25 % of patients (A-B: Courtesy of Quyen D. Chu, MD, MBA, FACS)
Due to the widespread availability and the fast, relatively simple acquisition of images, CT scan is the most frequently used imaging modality to detect pancreatic abnormalities. Although it is the most cost-effective imaging modality and provides excellent anatomic detail, it may be limited in its ability to detect small tumors or peritoneal metastases. CT scan should be performed according to a defined pancreas protocol that includes multiphase technique (arterial, parenchymal, portal venous) and thin slices (3 mm or less) through the abdomen.
Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) is an alternate imaging modality. However, it is no more accurate than CT in the diagnosis or evaluation of pancreatic cancer. Furthermore, it is not as widely used due to the decreased availability and slower acquisition of images. However, MRI benefits patients in that it avoids additional radiation exposure if further imaging is needed. An additional benefit is that it can be performed along with a cholangiopancreatography (MRCP) to provide more detailed views of the pancreatic and biliary ducts.
Endoscopic Ultrasound (EUS)
Endoscopic ultrasound (EUS) can be used for closer examination, biopsy, or staging of lesions in the vicinity of the head of the pancreas. Also, it may be complementary to CT for staging and is especially helpful in detecting small tumors, which are not always visible with other imaging modalities. EUS-directed fine-needle aspiration (FNA) is preferable to CT-guided FNA for resectable disease; this is due to the improved diagnostic yield, better safety profile, and lower risk of peritoneal seeding as compared with the percutaneous approach [9].
Endoscopic Retrograde Cholangiopancreatography (ERCP)
Endoscopic retrograde cholangiopancreatography (ERCP) can be used when a mass lesion cannot be identified by the above imaging modalities in a patient who requires further evaluation and sampling of the pancreatic duct for the workup of pain, jaundice, and/or pancreatitis.
Positron Emission Tomography (PET)
Positron emission tomography (PET) is a functional imaging modality. The role of PET/CT in pancreatic imaging is still evolving and, as such, is not widely used at this time for the diagnosis or follow-up of pancreatic cancers. It is not a substitute for high-quality, contrast-enhanced CT. However, PET/CT scans may be considered after formal pancreatic CT protocol in high-risk patients to detect metastases (Fig. 13.4).
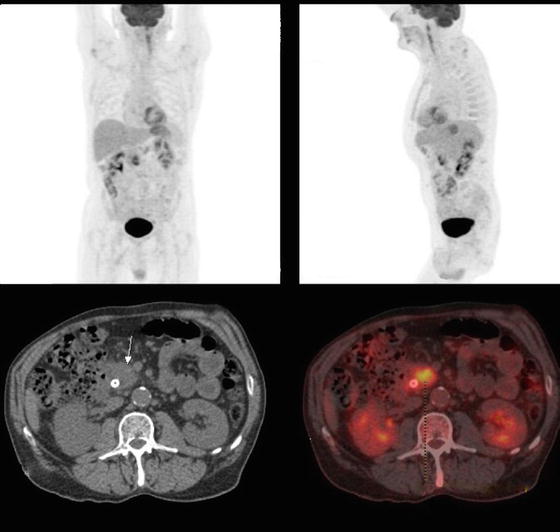

Fig. 13.4
A PET/CT scan of a 68-year-old male who presented with painless jaundice and a 3.0 cm mass in the head of the pancreas. There is a heterogeneously intense FDG avid mass involving the head of the pancreas that was suspicious for malignancy. There was no other FDG uptake identified elsewhere. The patient underwent a successful Whipple operation and the final pathology demonstrated a 4.0 cm pancreatic adenocarcinoma with 5/18 positive lymph nodes with evidence of cancer extending into portal vein. All margins, including the proximal and distal margin of the resected vein, were negative (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Diagnostic/Staging Laparoscopy
Recent improvement in the quality of CT imaging has decreased the utility of routine diagnostic laparoscopy (DL). However, laparoscopy is still used in many institutions prior to surgery or chemoradiation to rule out metastases, which may not be identified on imaging. This is especially true for lesions in the body and tail for which 50 % have evidence of peritoneal disease. DL is also helpful in selected cases, such as those that are at high risk for having disseminated disease (i.e., borderline resectable disease, patients with markedly elevated CA19-9 level, large primary tumors, or enlarged regional lymph nodes) [9]. DL identifies occult metastatic disease that is not otherwise detected by multiple imaging modalities. Progression of disease can be identified in up to 30 % of patients with diagnostic laparoscopy [12, 13].
Staging
There are two well-recognized staging systems for pancreatic adenocarcinoma: the first, and most commonly used, is the American Joint Committee on Cancer (AJCC), also known as the TNM staging system (Table 13.1); the second staging system is the National Comprehensive Cancer Network (NCCN). The AJCC/TNM staging system is also used by the Union for International Cancer Control (UICC) for clinical, surgical, and pathologic staging, as well as for following response after treatment. The NCCN criteria classify pancreatic adenocarcinoma on the basis of surgical resectability and as such are useful as a pretreatment staging system. NCCN guidelines were adopted and modified from an expert consensus statement spearheaded by pancreatic surgical societies including the American Hepato-Pancreato-Biliary Association (AHPBA), the Society for Surgery of the Alimentary Tract (SSAT), and the Society of Surgical Oncology (SSO) (Table 13.2).
Table 13.1
American Joint Committee on Cancer (AJCC) TNM staging for pancreatic cancer (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ* |
T1 | ≤2, limited to pancreas |
T2 | >2, limited to pancreas |
T3 | Beyond pancreas, but without involvement of the celiac axis or the superior mesenteric artery |
T4 | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) |
*This also includes the “PanInIII” classification | |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage IA | T1 | N0 | M0 |
Stage IB | T2 | N0 | M0 |
Stage IIA | T3 | N0 | M0 |
Stage IIB | T1-3 | N1 | M0 |
Stage III | T4 | Any N | M0 |
Stage IV | Any T | Any N | M1 |
Table 13.2
The National Comprehensive Cancer Network (NCCN) and the American Hepato-Pancreato-Biliary Association (AHPBA)/Society of Surgical Oncology (SSO)/Society for Surgery of the Alimentary Tract (SSAT) pretreatment staging system of pancreatic adenocarcinoma
Classification | Presurgical imaging criteria | Treatment recommended |
|---|---|---|
Localized and clearly resectable | Absence of distant metastases | Surgery followed by adjuvant chemoradiation or preoperative chemoradiation followed by surgery |
Clear fat planes around the CA, HA, and SMA | ||
No SMV/PV distortion | ||
Borderline resectable | Absence of distant metastases | Neoadjuvant therapy |
SMV/PV: | ||
Distortion or narrowing | ||
Occlusion but with suitable vessel proximal and distal, allowing for safe resection and replacement | ||
CHA: | ||
Abutment or short segment encasement | ||
CA: | ||
No abutment or encasement | ||
SMA: | ||
Abutment ≤ 1800 of artery circumference | ||
GDA: | ||
Encasement up to HA | ||
Locally advanced or unresectable | Absence of distant metastases | Chemoradiation |
Head: SMA encasement exceeding >180° | ||
CA abutment | ||
Unreconstructable SMV/PV occlusion | ||
Aortic or IVC invasion or encasement | ||
Body: | ||
SMA or CA encasement >180° | ||
Unreconstructable SMV/PV occlusion | ||
Aortic invasion | ||
Tail: | ||
SMA or CA encasement >180° | ||
Nodal status: metastases to lymph node beyond the field of resection | ||
Metastatic | Any evidence of distant metastases | Palliative treatment: non-operative, if possible |
NCCN criteria defining resectability status separate pancreatic tumors into three categories: (1) localized and clearly resectable, (2) borderline resectable, and (3) locally advanced or unresectable. The specific criteria for each category are listed in Table 13.2 [9]. Of note, the borderline resectable category was not introduced until 2006.
Treatment
The management of patients with pancreatic cancer requires a multidisciplinary approach that includes participation of medical, surgical, and radiation oncologists. This also requires the expertise of radiologists and pathologists. Proper initial evaluation of a patient presenting with a pancreatic mass includes a complete history and physical examination, along with a review of laboratory and imaging studies.
In order for the treatment of pancreatic cancer to be potentially curative, guideline-directed care includes both surgical resection and chemotherapy and chemoradiation as important modalities. The first step in surgical management of pancreatic cancer is appropriate selection of surgical candidates. A patient is considered to be a surgical candidate if all of the following criteria are met: (1) the patient has a surgical disease, (2) the disease is potentially curable (i.e., no evidence of distant disease), (3) the lesion is resectable (i.e., no encasement of the superior mesenteric artery (SMA), and (4) the patient has a good performance status.
Performance status can be determined using either the Karnofsky Performance Status (KPS) scale [14] or the ECOG/Zubrod Performance Status scale [15]; the latter is less cumbersome than the former. The ECOG/Zubrod scale ranges from 0 to 5 and is as follows: (1) Zubrod 0, asymptomatic; (2) Zubrod 1, symptomatic, fully ambulatory; (3) Zubrod 2, symptomatic, in bed less than 50 % of the day; (4) Zubrod 3, symptomatic, in bed more than 50 % of the day, but not bedridden; (5) Zubrod 4, bedridden; and (6) Zubrod 5, dead. The lower the ECOG/Zubrod scale, the lower the morbidity and mortality will be from surgery. In general, surgical candidates should have an ECOG/Zubrod 0–2.
On occasions, a patient may present with obstructive jaundice with or without a definitive lesion/mass in the periampullary/head of the pancreas region. If the patient has met all of the criteria to be a surgical candidate, a preoperative tissue biopsy by percutaneous means (i.e., CT-guided biopsy) should be avoided because of the risk of cancer seeding from the needle track. However, a preoperative endoscopic ultrasound-guided biopsy of the pancreas is an acceptable modality that carries a sensitivity and specificity reaching 90 % and 100 %, respectively [16]. Percutaneous biopsy such as a CT-guided biopsy can be considered if the patient is not a surgical candidate, and a tissue biopsy is required for further management counseling or if the patient is being considered for neoadjuvant therapy.
The definitions of resectability and borderline resectability are ever evolving, but as described above, current guidelines define resectable tumors as those characterized by the absence of distant metastases and possess clear fat planes around the celiac axis, hepatic artery, and SMA and no radiologic evidence of SMV or portal vein involvement [17] (Fig. 13.5). Pancreatic tumors are considered borderline resectable if there is partial involvement of the SMV or portal vein that would allow for safe resection and reconstruction, involvement of the GDA up to the hepatic artery with either only short segment encasement or direct abutment of the hepatic artery without extension to the celiac axis, and/or tumor abutment to the SMA not exceeding 180° of vessel wall circumference (Fig. 13.6).
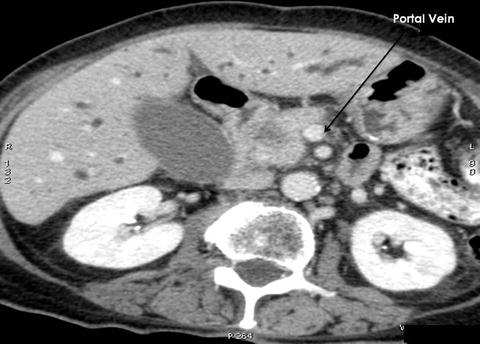
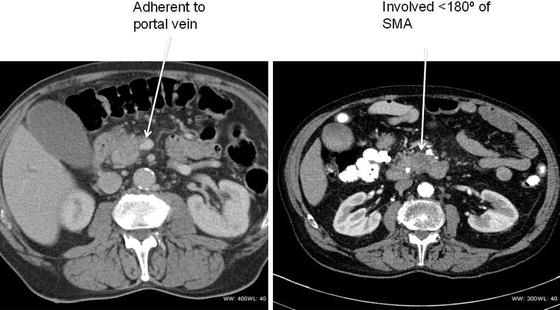

Fig. 13.5
CT scan of a patient with a mass in the head of the pancreas that is localized and easily resectable. Note a nice fat plane between the mass and the portal vein. A Whipple procedure demonstrated an adenocarcinoma of the head of the pancreas (Courtesy of Quyen D. Chu, MD, MBA, FACS)

Fig. 13.6
CT scans of patients with borderline resectable pancreatic cancer. Both patients underwent neoadjuvant therapy prior to a successful Whipple operation (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Standard management includes surgical resection first, followed by postoperative chemoradiation (adjuvant therapy) for those that are localized and clearly resectable. Chemoradiation is required because despite surgical resection, 80 % of patients have positive lymph nodes on pathology after a pancreatectomy, indicating that pancreatic cancer is often a systemic disease. For patients with borderline resectable disease, i.e., no clear margin between the tumor and the blood vessels, neoadjuvant treatment, which means chemoradiation first, followed by surgery if the tumor then becomes resectable, is often recommended. Increasingly complex vascular reconstructions and neoadjuvant therapies are increasing the boundaries of resectability; however, this must be taken in the context of the primary goal of surgical resection which is to completely remove any malignant cells. Patients left with residual disease at the time of operation have far worse outcomes than those patients with complete resections [18].
Surgical Resection
Early pancreatic resections were undertaken by surgical pioneers such as Trendelenburg, Billroth, Codivilla, Halsted, and Kausch in the late nineteenth and early twentieth centuries [19]. However, with perioperative mortality rates often exceeding 50 %, these operations were risky and often required multistage procedures. Operative indications and strategies were refined over the subsequent decades, culminating in the first one-stage pancreaticoduodenectomy with antrectomy performed by Dr. Allen Whipple in 1940 [20] and first total pancreatectomy by Dr. Eugene Rockey in 1942 [21]. Modest improvements in outcomes followed as surgical techniques improved over the ensuing decades, but perioperative mortality rates remained high, often exceeding 20 %, into the 1970s. However, the last 40 years has been marked by profound improvements in short-term (30-day) perioperative mortality. Mortality rates are closely associated with the extent of resection, with the highest risk occurring after a total pancreatectomy, the lowest risk occurring after a distal pancreatectomy, and an intermediate risk occurring after a pancreaticoduodenectomy (PD) [22].
Despite more recent improvements in surgical outcomes, especially at high-volume centers with perioperative mortality rates dropping below 2 % in some series [23], the historical trend of poor outcomes weighs heavy. This often leads to a nihilistic approach to the treatment of pancreatic cancer with many patients and physicians believing that the risks of pancreatic surgery outweigh the potential benefit.
Contradicting this viewpoint, recent literature suggests that as surgical techniques, patient selection, and high-volume centers continue to improve, so do surgical outcomes. Data from the Nationwide Inpatient Sample (NIS) demonstrated that the in-hospital mortality rate for all pancreatic cancer resections in the United States was 7.8 % in 1998, which was reduced to 4.6 % by 2003 [22]. Across the entire time frame of this study, the overall in-hospital mortality rate for distal pancreatectomy was 3.5 %, 6.6 % for PD, and 8.3 % for total pancreatectomy. There was a demonstrable persistent improvement in outcomes based on center volume, with a 2.4 % in-hospital mortality rate for high-volume hospitals versus 9.2 % for low-volume centers.
Despite steady gains in reducing perioperative mortality, pancreatic resections remain technically complex operations and therefore do still carry risks of significant complications including pancreatic fistula, anastomotic leak, delayed gastric emptying, infectious complications, bleeding, deep vein thrombosis, and cardiovascular events. Analysis of nationally representative discharge data from 1998 to 2006 identified a 22.7 % rate of major postoperative complications during the index hospitalization [24]. This rate remained stable, despite declining operative mortality.
As previously discussed, morbidity and mortality rates are closely correlated to the extent of the resection. Therefore, it is important to understand not only the technical aspects of each type of pancreatic resection but also the best approach for each patient with pancreatic cancer.
Pancreaticoduodenectomy
Also known as a Whipple procedure, pancreaticoduodenectomy is the most common curative resection performed for pancreatic adenocarcinoma confined to the head of the pancreas, including ampullary cancers. In this procedure, the head of the pancreas, the first and second portions of the duodenum, distal stomach, proximal jejunum, a portion of the common bile duct, the gallbladder, and the surrounding lymph nodes are removed (Fig. 13.7a, b). The reconstruction for gastrointestinal continuity includes connection of the jejunum to the remaining pancreatic duct (pancreaticojejunostomy), the bile duct (hepaticojejunostomy), and stomach (gastrojejunostomy) (“classic Whipple”; Fig. 13.7c).
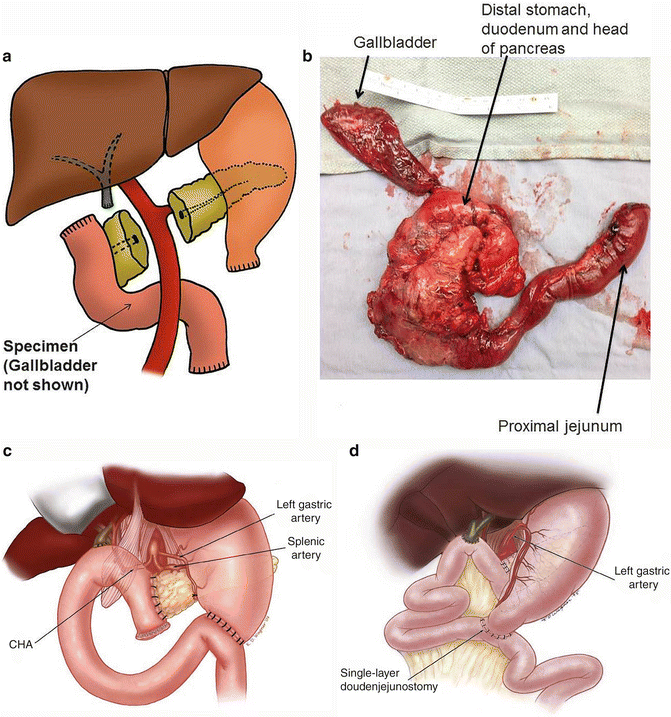

Fig. 13.7
Organs that are removed in a pancreaticoduodenectomy: a pancreaticoduodenectomy or a Whipple procedure removes the head of the pancreas, the first and second portions of the duodenum, a portion of the common bile duct, the gallbladder, and the surrounding lymph nodes. Reconstruction is achieved by performing a pancreaticojejunostomy, hepaticojejunostomy, and a gastrojejunostomy, “classic Whipple” (Figure 13. Alternatively, a pylorus-sparing reconstruction can also be performed) (A, B. Courtesy of Quyen D. Chu, MD, MBA, FACS) (C, D. Courtesy of Douglas B. Evans, MD, FACS)
“Classic” Versus Pylorus-Sparing Whipple Procedure
In comparison to the above-described “classic” Whipple procedure, the pylorus-sparing procedure does, as the name implies, preserve the stomach and pylorus. Instead, the very proximal portion of the duodenum is resected and the jejunum is then anastomosed to the duodenum (duodenojejunostomy) (Fig. 13.7d).
The debate surrounding the type of pancreaticoduodenectomy argues for pylorus-sparing on the basis of preserved gastric emptying on the one hand but, on the other hand, raises concerns about the oncologic resection, especially for larger tumors, thus advocating for the “classic” approach. Studies, however, have demonstrated no significant differences in outcomes between the two variations of this procedure. Tran et al. examined patients undergoing resection for suspected pancreatic or periampullary cancers and found no differences in median blood loss (p = 0.70), duration of the operation (p = 0.10), incidence of delayed gastric emptying, or overall survival between the “classic” approach and pylorus-sparing approach [25]. In a similar randomized study, Seiler et al. found that morbidity, long-term survival, quality of life, and weight gain were identical between these two variations of the procedure [26].
Total Pancreatectomy
Depending on the extent of the cancer, total pancreatectomy may be required for complete resection (Fig. 13.8). In this procedure, the entire pancreas and spleen are removed. Removing the entire pancreas, and thereby all of the insulin-producing islet cells, renders the patient to be a brittle diabetic after this procedure. Completion total pancreatectomy is often an option for patients who are septic from severe disruption of the pancreaticojejunostomy anastomosis following an elective Whipple procedure or life-threatening hemorrhage that is not amenable to conservative treatment [27].
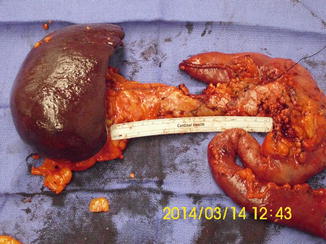

Fig. 13.8
Total pancreatectomy: a 56-year-old Caucasian man presented with a significant family history of pancreatic cancer. He insisted on having a screening CT scan, which revealed two separate lesions in the tail and head of the pancreas. Despite multiple counseling, he opted for a prophylactic total pancreatectomy. Final pathology demonstrated both lesions to be intraductal papillary mucinous neoplasms (IPMN) (Courtesy of Gazi Zibari, MD, FACS)
Distal Pancreatectomy
For tumors confined to the body or tail of the pancreas, distal pancreatectomy may be performed. This procedure can be performed with or without splenectomy; most commonly it is performed with splenectomy given the overlapping blood supply and lymphatic drainage (Fig. 13.9a–b). Appropriate vaccines covering encapsulated bacteria such as Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis or meningococcus should be given at least 2 weeks prior to surgical resection.
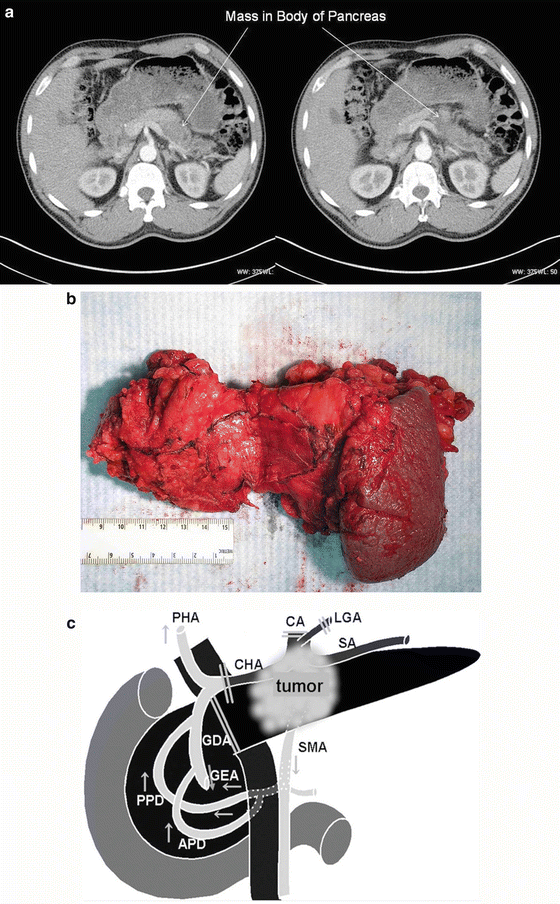

Fig. 13.9
CT scan of a patient with a mass in the body of the pancreas (a). The patient underwent a distal pancreatectomy and a splenectomy, which demonstrated an adenocarcinoma (b). The tumor measured 4.0 cm in maximal diameter with 2 out of 35 involved lymph nodes. Margins of resection were all negative. Schematic drawing of tumor involving the body of the pancreas and the celiac axis that requires a distal pancreatectomy/splenectomy and resection of the celiac axis (Appleby operation) (Fig. 13.9c). Note that retrograde flow to the proper hepatic artery (PHA) is via the gastroduodenal artery (GDA). APD anterior pancreaticoduodenal arcade, CA celiac axis, CHA common hepatic artery, GEA right gastroepiploic artery, LGA left gastric artery, PHA proper hepatic artery, PPD posterior pancreaticoduodenal arcade, SA splenic artery, SMA superior mesenteric artery (A–B. Courtesy of Quyen D. Chu, MD, MBA, FACS) (C. Reprinted from Hirano S, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: Long-term results. Ann Surg 2007;246 (1):46–51 with permission from Lippincott Williams & Wilkins)
The common hepatic artery, the origin of the splenic artery, and/or the celiac axis can often be involved with locally advanced cancer following neoadjuvant therapy. Although controversial, in such a patient who has no evidence of distant disease, some surgeons would perform a modified Appleby procedure. The original Appleby procedure was described in the 1950s in a patient with advanced gastric cancer who underwent a total gastrectomy, distal pancreatectomy/splenectomy, and celiac axis resection [28]. The modified Appleby excludes gastrectomy. Following ligation of the celiac axis, flow to the liver is established via retrograde flow from the gastroduodenal artery (Fig. 13.9c). The procedure, although rarely done, is mentioned only to provide the readers with a better appreciation of the anatomy of the region.
Surgical Considerations
Portal Vein Reconstruction
For cases in which the pancreatic tumor involves the superior mesenteric vein (SMV), portal vein (PV), or both, SMV/PV resection may be necessary to achieve negative margins (Fig. 13.10a, b). This should only be undertaken in special circumstances in which it is believed that R0 (microscopically negative) or R1 (grossly negative, but microscopically positive) margins can be achieved. It should also only be performed by experienced surgeons. Additional criteria for performing such a complex endeavor require that the patient has adequate inflow and outflow of the reconstructed veins and lacked any involvement of the superior mesenteric artery (SMA) and hepatic artery [29].
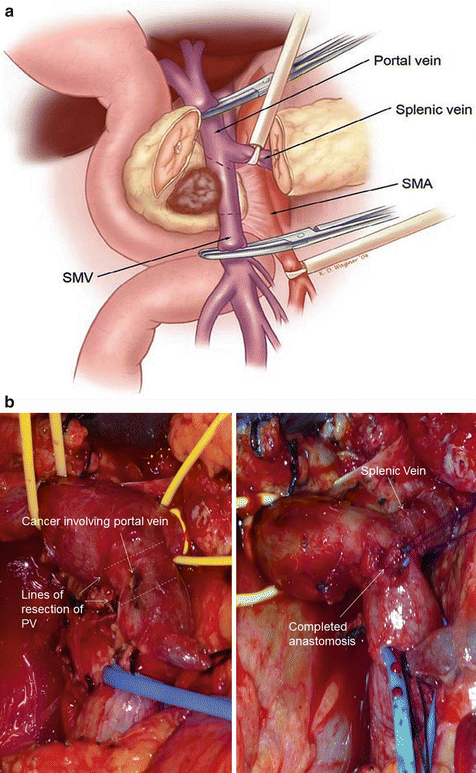

Fig. 13.10
Technique of performing a portal vein resection (a). Note that vascular control needs to be achieved at the level of the splenic vein, proximal and distal portion of the portal vein, and any other tributaries draining the resected portion of portal vein. Intraoperative pictures of a portal vein resection with primary anastomosis (b) (A. Courtesy of Douglas B. Evans, MD, FACS) (B. Courtesy of Quyen D. Chu, MD, MBA, FACS)
Extended Lymphadenectomy
This technique is currently debated as there is no definitive evidence that extended lymphadenectomy, which involves resection of retroperitoneal lymph nodes, improves survival [30]. Retrospective reports and smaller randomized trials have suggested some increase in survival, but this is not a consistent finding. Therefore, it is generally recommended, except for rare instances, such as part of a clinical trial or in the case of a large tumor that requires extended lymphadenectomy as part of an en bloc resection, that routine extended lymphadenectomy should not be performed [31].
Preoperative Biliary Drainage/Stent
Often patients with pancreatic cancer, especially those with pancreatic head tumors, present with obstructive jaundice. Symptoms of obstructive jaundice can range from mild to severe and may cause such complications as hepatic dysfunction, coagulopathy, pruritus, and cholangitis [32, 33]. In cases of severe symptoms and/or such complications, decompression of the biliary system may be required, which can successfully be accomplished with either endoscopic or percutaneous biliary stent placement. In more mild cases, the routine use of biliary drainage/stenting in the preoperative setting remains controversial. The initial rationale for decompression in these cases arose from early clinical experience that jaundiced patients undergoing surgical resection were at risk for developing postoperative complications such as infection, bleeding, and renal failure [34]. However, a more recent multicenter, randomized trial comparing preoperative biliary drainage with surgery alone for patients with cancer of the head of the pancreas found that the preoperative biliary drainage group had a significantly higher complication rate (74 %) than the surgery-only group (39 %; p < 0.001) [35]. Therefore, current recommendations for consideration of preoperative biliary drainage/stent include patients with the following symptoms/complications—cholangitis, severe intractable pruritus, and coagulopathy—and those who will not undergo immediate surgical resection [32, 33]. Self-expanding metallic stents are preferred over plastic ones [36].
Postoperative Pancreatic Fistulas (POPF)
Although mortality after a pancreaticoduodenectomy is acceptably low (0–5 %), morbidity remains substantial, ranging from 32 % to 52 % [37–39]. Of the complications, postoperative pancreatic leak/fistula (POPF) is one of the most dreaded. Depending on how one defines POPF, leakage rate varies from 0 % to 25 % [40]. Sequelae of this serious complication includes delayed gastric emptying (DGE), abdominal hemorrhage, and abscess, the latter two can result in a mortality rate of 40 % or more [41, 42].
There are many different ways of managing the pancreatic stump following a pancreaticoduodenectomy (i.e., pancreatic duct occlusion, trans-anastomotic stenting, somatostatin, etc.), although there is no consensus on any one best way. Risk factors associated with pancreatic leak following a Whipple procedure can be categorized into those that are disease-related, patient-related, and operative-related (Table 13.3).
Table 13.3
Risk factors for developing pancreatic fistula following a Whipple procedure
Organ/disease–related factors |
Soft pancreatic parenchyma |
Duct ≤ 3 mm diameter |
Pancreatic pathologies |
Ampullary or duodenal carcinoma |
Distal cholangiocarcinoma |
Intraductal papillary mucinous neoplasia (IPMN) |
Pancreatic cystadenomas |
Benign islet cell tumors |
Duodenal adenomas |
Patient–related factors |
Male gender |
> 70 years of age |
Prolonged jaundice |
Creatinine clearance abnormality |
High intraoperative blood loss (>1,000 ml) |
Coronary artery disease |
Operative–related factors |
High intraoperative blood loss (>1,000 ml) |
Prolonged operative time |
Surgeon’s inexperience |
Examples of disease-related factors include a soft pancreas because, unlike a fibrotic pancreas, pancreaticojejunostomy anastomosis is more difficult to perform. Other disease-related factors include a pancreatic duct less than or equal to 3 mm in diameter, absence of parenchymal fibrosis, fatty infiltration of the pancreatic parenchyma, and resection of pathologic entities that lacked a fibrotic pancreas such as ampullary or duodenal cancer, intraductal papillary mucinous neoplasia, cystadenomas, benign islet tumors, duodenal adenomas, and distal cholangiocarcinoma [43].
Patient-related risk factors include male gender, age > 70 years, high body mass index (BMI), coronary artery disease, prolonged jaundice, and creatinine clearance abnormality. Operative-related factors include high intraoperative blood loss (>1,000 ml), prolonged operative time, and surgeon’s inexperience.
Some investigators proposed that a pancreaticogastrostomy instead of a pancreaticojejunostomy and a duct to mucosa rather than invagination of the jejunum into the pancreas are factors that decrease the rate of POPF. However, there is no definitive data to suggest that one reconstructive technique is better than another, and the selection of a reconstructive technique should be left at the discretion of the surgeon. Of interest, diabetes mellitus and neoadjuvant chemoradiation therapy appear to be protective against POPF. The mechanism is unknown, but it is thought that chemoradiation causes a reduction in pancreatic exocrine excretion, thus, leading to a lower rate of POPF.
POPF rate varies, depending on how POPF is defined. The International Study Group of Pancreatic Fistula (ISGPF) developed a grading system to standardize the definition of POPF so that surgical experiences among centers can accurately be compared [44] (Table 13.4). PFs are defined as high amylase content from the abdominal drain (>3 times the upper normal serum value) at any time on or after the third postoperative day and are grouped into grade A, B, or C based on nine clinical criteria. Grade A fistula is the most common and often referred to as a “transient fistula”; it is a biochemical leak without clinical significance and is self-limited that does not require significant intervention. However, grades B and C are clinically relevant fistulae that may require significant intervention. Grade B fistula can be associated with fever, leukocytosis, and abdominal pain. The patient’s abdominal drain is left in place for a prolonged period while he/she is generally placed on parenteral or enteral nutrition. Grade C fistula can result in a life-threatening event. These patients may suffer multiorgan system failure, despite aggressive conservative management. Patients who decompensate from a significant POPF may require a reoperation to undergo a conversion of a pancreaticojejunostomy to pancreaticogastrostomy, a repair of the leak with wide peripancreatic drainage, or a completion total pancreatectomy [44, 45].
Table 13.4
International Study Group of Pancreatic Fistula (ISGPF) grading of postoperative pancreatic fistula (POPF)
Criteria | Grade A fistula | Grade B fistula | Grade C fistula |
|---|---|---|---|
Drain amylase level | >3 times normal serum amylase | >3 times normal serum amylase | >3 times normal serum amylase |
Clinical conditions | Well | Often well | Ill appearing/bad |
Specific treatmenta | No | Yes/no | Yes |
US/CT (if obtained) | Negative | Negative/positive | Positive |
Persistent drainage (>3weeks)b | No | Usually yes | Yes |
Reoperation | No | No | Yes |
Death related to POPF | No | No | Possibly yes |
Signs of infection | No | Yes | Yes |
Sepsis | No | No | Yes |
Readmission | No | Yes/no | Yes/no |
Role of Prophylactic Somatostatin
Concerns about pancreatic fistula have prompted several investigators to evaluate the efficacy of prophylactic somatostatin in reducing such a dreaded complication. The use of prophylactic somatostatin analogues to reduce POPF remains controversial. It has not been shown to reduce mortality, and recently, a number of investigators are cautioning against its routine use. At least three meta-analyses were performed on the role of prophylactic somatostatin, one of which demonstrated no benefit in preventing clinical anastomotic leak [46–48]. A recent Cochrane analysis of 21 trials found that although the overall postoperative complication rate was significantly lower in the prophylactic somatostatin group, there were no significant differences in the reoperation rate, length of hospital stay, incidence of clinically significant fistula, or mortality rate between the somatostatin group and the controlled group [48]. In a group of patients who underwent a pancreaticoduodenectomy for malignancy, there was no significant difference in the pancreatic leak rate between the groups that received octreotide and the group that did not [49]. The differences in the conclusions of the meta-analyses may be due to publication selection biases and heterogeneity in endpoints. Somatostatin might be useful in selective cases such as those that are at risk for developing a POPF (i.e., soft gland, small duct, excessive intraoperative blood loss) [41]. Prophylactic somatostatin did not appear to have an impact on grade A fistulas [41].
Trans-anastomotic Pancreatic Duct Stenting
Trans-anastomotic pancreatic duct stenting has been proposed as a method to reduce the incidence of POPF. The theoretical advantage with stenting is that it diverts pancreatic juice away from the pancreaticojejunal anastomosis to avoid a leak. A randomized trial comparing external stenting versus no stenting found that the former had a significantly lower POPF rate (26 % vs 42 %; P = 0.034), morbidity rate (42 % vs 62 %; P = 0.01), and delayed gastric emptying rate (7.8 % vs 27 %; P = 0.001), although mortality was not impacted (3.7 % vs 3.9 %; P = 0.37) [50]. A recent meta-analysis of seven trials comparing externalized stent versus no stenting confirmed the above findings [51]. The same meta-analysis also found that when comparing internal stenting to no stenting, there was no significant difference between the two groups in postoperative complication rate. However, two randomized trials found equivalent outcomes between external and internal stenting, suggesting the role for internal stenting [52, 53]. The design of the clinical trials was very different (i.e., some perform a duct-to-mucosa pancreaticojejunostomy, while others do not) such that it is difficult to draw a definitive conclusion on the role of stenting. The decision of whether or not to stent and the choice of stenting technique are best left to the operating surgeon.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree