Fig. 11.1
Cell lineage schematic for pancreatic development from a multipotent progenitor stem cell
Basic Anatomy
The human pancreas is a long tapered glandular organ, which lies in the retroperitoneum. It is divided into four anatomical regions, the head, neck, body and tail, along with one accessory lobe or ‘uncinate process’, with the head lying within the curve of the second part of the duodenum. It extends transversely towards the hilum of the spleen, measuring between 12 and 15 cm long in adults. The digestive enzymes and bicarbonates are secreted by the exocrine acinar tissue. The acini (the name stems from the Greek word ‘acinus’ meaning grape) are an accumulation of acinar cells at the termination of ducts, and then drain into a centrally located acinar spaces connected to tiny pancreatic tubular ductal networks. These networks eventually join to form the larger pancreatic ducts namely the main pancreatic duct of Wirsung, and the accessory duct of Santorini. The main duct drains into the duodenum via the major duodenal papilla (Ampulla of Vater), while the main pancreatic duct may have a separate accessory pancreatic duct (derived from proximal duct of Santorini), which drains the uncinate process and lower part of the head of the pancreas into the duodenum via the minor duodenal papilla. Incomplete stet of the dorsal and ventral pancreatic ducts results in pancreas divisum, which is the most common congenital pancreatic ductal anatomic variant, but many anatomical variations of the pancreatic ductal drainage system do exist (Fig. 11.2). Blockage of the main pancreatic duct can lead to stasis of the digestive enzymes, which can then become activated and begin an auto-digestion process within the pancreas, resulting in pancreatitis. The endocrine hormones are produced in the islets of Langerhans, which are scattered throughout the pancreas, drained by a network of capillaries that invade the islet, and are thus delivered into the main bloodstream [2]. The distribution of endocrine cells within the islet is species-dependent. In rodents, the core of the islet is occupied by the β-cells surrounded in the periphery by a ring of α-cells, whereas in humans and monkeys all the endocrine cell types are intermingled with each other [3]. Non-endocrine cells also exist in the islet, including endothelial cells, neurons, dendritic cells, macrophages, and fibroblasts (Fig. 11.3).
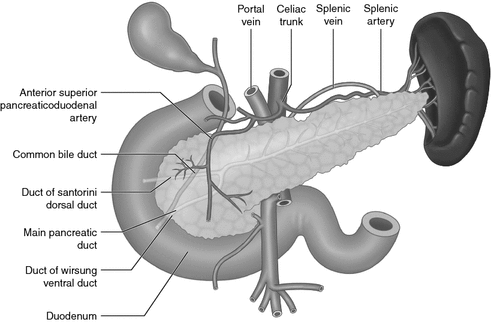
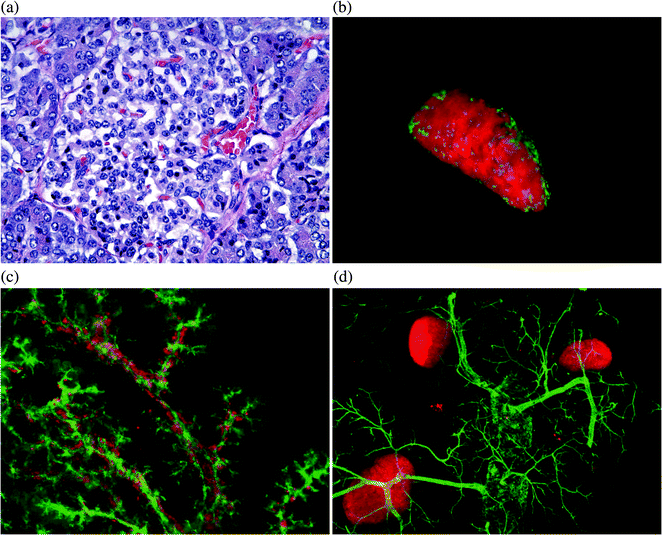

Fig. 11.2
Schematic illustration of the anatomical relationship of the pancreas in relation to other anatomical structures. The pancreatic head lies in front of the IVC and SMV, whereas the uncinate process lies posterior to the SMV. The stomach lies anterior to the pancreas, whereas the aorta, left adrenal gland and left kidney lie posterior to the body of the pancreas. The tail lies in the hilum of the spleen with the splenic artery running along the superior border of the pancreas. Major blood supply for the pancreas arises from the celiac trunk and superior mesenteric arteries, with the superior pancreaticoduodenal artery running anterior to head of pancreas. Innervation of the pancreas is derived from the vagal and splanchinic nerves. The pancreas is drained by multiple lymph node groups. The body and tail drain mostly into pancreatico-splenic nodes, whereas the head and neck drain more widely into nodes along the superior mesenteric, hepatic and pancreaticoduodenal arteries

Fig. 11.3
Standard histological section of a human pancreas specimen, a illustrating a small, relatively pale-staining cell known as the islet of Langerhans, which is embedded in darker-stained exocrine tissue. b Adult mouse pancreas wholemount image showing an isolated islet stained with insulin, with glucagon in the periphery. c Wholemount image of embryonic day (E) 16.5 pancreas illustrating close relationship between insulin cells and pancreatic ducts. d Wholemount image of an adult mouse pancreas stained with insulin and DBA for pancreatic ducts
A sound knowledge of the anatomical relationship of the pancreas to other surrounding structures is important when performing pancreatic surgery. The pancreatic head lies in front of the inferior vena cava, right renal artery, both renal veins and the superior mesenteric vessels, whereas the uncinate process lies posterior to the superior mesenteric vessels. The neck of the pancreas lies directly over the portal vein and vertebral bodies L1 and L2; this region is where the splenic vein unites with the superior mesenteric vein to form the portal vein. The splenic vein receives the inferior mesenteric vein near the tail of the pancreas. Anterior–posterior blunt trauma can thus lead to pancreatic tissue damage as well as ductal injury. The common bile duct passes in a deep groove on the posterior aspect of the pancreatic head until it joins the main pancreatic duct at the ampulla of Vater in the pancreatic parenchyma. The stomach lies anterior to the body and tail of the pancreas, whereas the aorta, left adrenal gland and left kidney lie posterior to the body of the pancreas. The tail lies in the hilum of the spleen with the splenic artery, which is often tortuous, running along the superior border of the pancreas (Fig. 11.2).
The major blood supply to the pancreas arises from multiple branches of the celiac trunk and superior mesenteric arteries, which form arterial arcades within the body and tail of the pancreas. The splenic and common hepatic arteries arise from the celiac trunk. The dorsal and greater pancreatic arteries branch from the splenic artery, whereas the gastroduodenal artery branches from the common hepatic artery, then dividing around the head of the pancreas into anterior and posterior superior pancreaticoduodenal branches that anastomose with the anterior and posterior branches of the inferior pancreaticoduodenal artery, which are branches of the superior mesenteric artery (Fig. 11.2). The vascularization of the pancreatic islets will be discussed in greater detail later in the chapter. Venous drainage of the pancreas mainly flows into the portal system, with the head and neck draining primarily through the superior and inferior pancreaticoduodenal veins, whereas the body and tail drain into the splenic vein.
Physiology
The pancreas is responsible for regulating the body’s glycemia through the endocrine cells within the islets of Langerhans. Islets constitute nearly 2% of the total pancreatic tissue. This regulation is delicately balanced through the actions of the hormones insulin and glucagon. Insulin is the hormone of energy storage, and induces an increase in amino acid uptake as well as glucose uptake, increasing protein synthesis, decreasing lipolysis and glycogenolysis, especially post-prandially or in a hyperglycemic state. Whereas glucagon is viewed as the hormone of energy release, stimulating higher blood glucose levels by the stimulation of gluconeogenesis, glycogenolysis and lipolysis in the setting of hypoglycemia.
β-cells secrete insulin based on blood glucose levels as well as neural and humoral factors. The stimulus for insulin release into the bloodstream is far greater when glucose is ingested enterally compared to the parenteral route, indicating that a ‘feed-forward’ mechanism in the digestive tract is activated, anticipating the rise in blood glucose. This anticipation is mediated by incretins. There are two main incretin hormones, glucose-dependent insulinotropic peptide; also known as gastric inhibitory peptide (GIP) and glucagon-like peptide-1 (GLP-1). Both are secreted by endocrine cells located in the small intestinal epithelium when the luminal concentration of glucose increases in the digestive tract, and subsequently they stimulate the β-cells to secrete ‘more’ insulin. Hence, the great interest in the pharmaceutical industry to develop incretin-based therapies to treat diabetes, particularly type 2, because of its potent secretagogue effect on β-cells. Unlike traditional medications that stimulate β-cells to secrete insulin regardless of blood glucose level, incretins augment the β-cell response to blood glucose levels in a glucose-dependent manner, in addition to GLP-1’s inhibitory effect on glucagon secretion and the ability to increase food transit time in the stomach [4, 5]. A peculiar phenomenon observed is type 2 diabetic patients undergoing Roux-en-Y (RYGBP) surgery is that they experience a dramatic amelioration of blood glucose homeostasis and insulin sensitivity even before weight loss. The underlying mechanism behind this dramatic improvement is not fully understood. The dumping of nutrients into the distal small intestine stimulates an exaggerated GLP-1 peptide release from intestinal endocrine L-cells into the portal vein, stimulating insulin release. Humoral inhibitors for insulin release include somatostatin, amylin, leptin and pancreastatin. Vagus nerve generally stimulates insulin release, whereas the sympathetic nervous system inhibits it, mediated by various peptidergic molecules secreted from nerve fibers such as substance P, VIP, and neurotensin.
The exocrine pancreas on the other hand consists of two morphologically distinct structures. Acinar cells, the functional unit of the exocrine pancreas which secrete digestive enzymes, and duct cells that mainly secrete bicarbonate-rich fluid. The total external secretion of the pancreas consists of clear, colorless, bicarbonate-rich alkaline solution of about 2.5 L per day. Exocrine secretion is stimulated by the hormones secretin and cholecystokinin (CCK), and by parasympathetic vagal discharge. Serum amylase is usually measured to diagnose pancreatitis, which is usually 2.5× normal within 6 h after the onset of an acute episode, and then returns to normal within 3–7 days. However, the major limitation with the use of serum amylase measurement to diagnose pancreatitis is the lack of specificity as several clinical conditions can result in an elevated amylase. In addition, a normal serum amylase certainly does not exclude pancreatitis. The amylase-to-creatinine ratio (ACR) may help in differentiating acute pancreatitis from other conditions using the following equation:


An ACR greater than 5% suggests acute pancreatitis, and ratios less than 1% suggest macroamylasemia. Serum lipase levels, on the other hand, are thought to be more specific in diagnosing pancreatic tissue damage because it is only produced in the pancreas. Lipase tends to be higher in alcoholic pancreatitis and the amylase level higher in gallstone pancreatitis, hence the lipase-to-amylase ratio has been suggested as means to distinguish between the two.
Imaging
There are several imaging modalities that are useful for investigating pancreatic disease overall. Each modality has its advantages and drawbacks. Often a combination of methods is required for the accurate diagnosis of conditions of the endocrine pancreas.
Plain Abdominal Radiographs
Although not useful for directly imaging the pancreas, plain abdominal radiographs may indirectly reveal an inflamed pancreas, demonstrating a ‘sentinel loop’, which is an isolated bowel segment (jejunum, transverse colon or duodenum) that is dilated next to the inflamed pancreas. Plain radiographs may also reveal the ‘colon cutoff sign’. This sign represents the abrupt termination of gas within the proximal colon at the level of the radiographic splenic flexure which is caused by colonic spasm secondary to pancreatic inflammation. It must be noted however, that both signs are nonspecific for pancreatitis. In the setting of endocrine insufficiency in chronic pancreatitis, calcification may be visualized. Patients may also develop pleural effusions which can be seen on chest X-rays. Structural abnormalities such as annular pancreas may be associated with duodenal obstruction/atresia resulting in the classic ‘double-bubble’ sign on an abdominal X-ray.
Abdominal Ultrasonography (US)
US is a helpful imaging modality to examine the pancreas in pediatric patients, although overlying bowel gas can make imaging variable. It can be used as a simple, noninvasive test for diagnosing a mass within the pancreas, and can demonstrate a peri-pancreatic fluid collection and edema.
Abdominal Computed Tomography (CT) Scans
CT scans offer much better resolution of the pancreas than US in terms of assessing the size and location of an endocrine tumor within the pancreas or a tumor within the gastrinoma triangle. It is the imaging of choice for assessing complications of pancreatitis but it should be noted that inflammatory changes are not usually radiographically present during the first 72 h of pancreatic inflammation [6].
Endoscopic Retrograde Cholangiopancreatography (ERCP)
ERCP is increasingly being used in children with pancreaticobiliary disease, both as a diagnostic procedure and therapeutic tool, with complication rates similar to that seen in adults [7]. As a result, ERCP is now considered a safe and useful tool for use in children in the hands of a well-trained endoscopist.
Magnetic Resonance Cholangiopancreatography (MRCP)
MRCP is a noninvasive and safer test compared with ERCP in diagnosing choledocholithiasis in the setting of pancreatitis, especially if there is real concern that ERCP may worsen pancreatitis. Although it is not as sensitive as ERCP, with sensitivity reported as low as 38%. However, the use of secretin has shown very promising results with sensitivity and specificity approaching 98% up to 100% in assessing patients with pancreatic divisum or common bile duct obstruction, respectively [7]. It is now the initial imaging of choice in evaluating pancreatic ductal anatomy in children with unexplained or recurrent pancreatitis [8].
Other Imaging Modalities for the Endocrine Pancreas
Many researchers are looking into developing simple, noninvasive methods to image the β-cells in vivo in order to measure their mass, function, severity of disease and inflammation. With recent advances in imaging technology such as magnetic resonance imaging (MRI), positron emission tomography (PET), ultrasound backscatter microscopy, and fluorescence spectroscopy, this is starting to be a reality. Type 1 diabetics have a decrease in β-cell mass due to autoimmune destruction whereas type 2 patients have initially an increase in β-cell mass due to insulin resistance, but the increase can no longer compensate for the increase in insulin demand. Currently, the only true marker of β-cell mass in vivo that can be realistically used is insulin (or the pro-insulin by-product C-peptide). However, more than 90% of β-cells must be lost in order to detect inappropriate decreases in insulin and C-peptide levels relative to glucose levels. So essentially, no useful in vivo islet imaging modality currently exists, which is also due to other logistical problems such as the fact that islets make up only 2% of the whole pancreas, which lies in a retroperitoneal position.
Presently, researchers are looking at certain surface markers on β–cells, which could logically serve as a guide to measuring β–cell mass, such as glucose transporter-2 and glucagon-like peptide-1 receptor which are significantly downregulated during hyperglycemia. In addition, unique β-cell metabolism may provide a target for PET scans to target lymphocytes which infiltrate islets in type 1 diabetic patients. These lymphocytes may be tagged with an imaging contrast agent to monitor inflammation, and antibody-guided targeting of ultrasound-detectable micro-bubbles aggregating in the islet microcirculation to allow for sufficient β-cell mass imaging to name but a few. The ultimate aim is to develop a simple and reliable bedside method for monitoring disease progress and measuring the clinical response to therapy in diabetic patients.
Basic Pancreatic Embryology
The embryonic pancreas is known to pass through three stages of development [9]. The first is the undifferentiated stage where the endoderm evaginates to initiate pancreatic morphogenesis, with only insulin and glucagon genes being expressed at this stage [10]. The second phase involves epithelial branching morphogenesis with simultaneous formation of primitive ducts. This stage involves the separation of islet progenitors beginning to differentiate and losing their attachments to the basement membrane [11]. The third and final stage begins with the formation of acinar cells at the apices of the ductal structures, with the development of zymogen granules containing enzymes. Acinar cells usually commence enzyme secretion shortly after birth [9, 12].
Organogenesis of the pancreas is initiated with the regional specification of the undifferentiated primitive foregut tube by transcription factors pancreatic and duodenal homeobox 1 (Pdx1), which marks the pre-pancreatic endoderm and by pancreas transcription factor (PTF1a), where both are expressed in multipotent pancreatic progenitor cells [13]. The first appearance of the pancreas appears morphologically as a mesenchymal condensation at the level of the duodenal anlagen, distal to the stomach on the dorsal aspect of the foregut tube at embryonic day (E) 9.0 in mice. All cells expressing Pdx1 and PTF1a in the endoderm will eventually give rise to all of the epithelial cells in the adult pancreas, which includes endocrine, acinar, and duct cells [1]. At around E9.5 gestation in mice and the twenty-sixth day of gestation in humans, the dorsal bud begins to evaginate into the overlying mesenchyme while retaining luminal continuity with the gut tube [14]. Approximately 12 h later in mice, and six days after dorsal bud evagination in humans, the ventral bud begins to arise. Gut rotation will bring the ventral lobe dorsally, ultimately fusing with the dorsal pancreatic bud (this event corresponds to around the sixth to seventh week of gestation in humans or E12-E13 in mice) contributing to the formation of the uncinate process and inferior part of the head of the pancreas, while the rest of the pancreas arises from the dorsal pancreatic bud. The entire ventral pancreatic duct and the distal part of the dorsal pancreatic duct fuse together to form the main pancreatic duct of Wirsung. The remaining proximal part of the dorsal pancreatic duct is either obliterated or persists as a small accessory pancreatic duct of Santorini [15]. This fusion of the two buds is followed by elongation of the pancreatic bud stalk region (precursor to the main pancreatic duct) and branching morphogenesis of the apical region of the bud. Unlike the usual branching morphogenesis growth patterns seen in the developing kidney, lung, and salivary gland, in which the branching morphogenesis occurs at 90° angles, the pancreas grows in an acute-angled branching pattern, which leads to the exclusion or ‘squeezing out’ of mesenchyme from between the closely apposed branches of epithelium (Fig. 11.4). This exclusion of mesenchyme may influence epithelial–mesenchymal interactions and lineage selection. The pancreas then undergoes major amplification of the endocrine cell population through two distinct waves of differentiation within the pancreatic epithelium during embryogenesis, an early primary wave (pre E13.5 in mice), followed by the secondary wave of differentiation (E13.5-E16.5 in mice) [1]. Over a similar gestational window the exocrine pancreatic precursors undergo an exponential increase in branching morphogenesis and acinar cell differentiation.
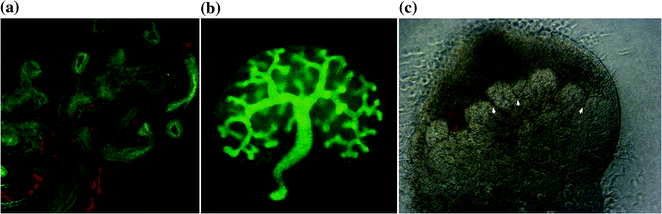

Fig. 11.4
Pancreatic branching morphogenesis is different than other organ systems such as lung and kidney. a Wholemount of E12.5 lung stained for Ecad, PGP9.5 (a neuronal marker) and CD31. b E11.5 kidney cultured for 3 days and stained with the epithelial marker Calbindin-D28 k, both demonstrating 90° branching pattern. c E11 whole pancreas from CD-1 cultured for five days revealing acute branching pattern mesenchymal exclusion zones (arrow-head). Mesenchyme contains factors that regulate pancreatic growth and differentiation
Dorsal and Ventral Pancreatic Bud Development
It is important to note that while the morphological development of the ventral and dorsal pancreatic buds may be similar, they differ markedly at the molecular level, with various lines of evidence suggesting that there are differences in the specification between both pancreatic rudiments, with the notochord playing a key role. Sonic hedgehog (Shh), which is a potent intercellular patterning molecule, is expressed along the entire foregut, is noticeably suppressed in the prospective pancreatic endoderm. This suppression of Shh appears to be necessary for dorsal pancreatic development, permitting the expression of pancreas specific genes including pdx1 and insulin. Deletion of the notochord in chick embryo cultures leads to ectopic Shh being seen in the pancreatic region of the foregut endoderm, with subsequent failure of the pancreas to develop [16]. Furthermore, the pancreatic anlage normally remains in contact with the notochord in mice until the paired dorsal aortae fuse in the midline (~E8.0). Activin-βB (a member of the transforming growth factor-β family) and FGF2 (fibroblast growth factor) both mimic notochord activity in inducing pancreatic genes [16]. In stark contrast to the dorsal bud, developmental gene expression in the ventral pancreatic anlage is not affected when the notochord is removed [17]. It appears that ventral pancreatic bud development is under the control of signals from the overlying cardiogenic mesenchyme, which also produces pro-hepatic signals (FGFs) to induce liver formation. Lack of pro-hepatic FGF signaling in regions of the cardiogenic mesenchyme will lead to the endoderm by ‘default’ differentiating into ventral pancreas [18]. When ventral foregut endoderm is cultured in the absence of cardiac mesoderm or FGF, it fails to activate liver specific genes, with instead pdx1 being expressed. Cardiac mesoderm, through FGF, induces liver formation from the ventral endoderm, and simultaneously inhibits pancreatic development [18]. Further differences between ventral and dorsal pancreas are demonstrated in Hlxb9 mutant mice. The homeobox gene Hlxb9, which is transiently expressed in the endoderm in the region of the dorsal and ventral pancreatic anlage, when inactivated in mice, only dorsal pancreatic development is blocked [19]. Hex is an early marker of the anterior endoderm [20] and is expressed at E7.0 in the cells that will subsequently gives rise to the ventral pancreas and liver. Hex null mutant embryos have specific failure of ventral pancreatic bud development, with the dorsal bud developing normally [21]. These examples underscore the significantly different molecular controls governing dorsal and ventral pancreatic bud development.
Initiation of the Pancreas with Endodermal Patterning
The signaling molecules that govern the specification of the primitive gut tube into different specialized domains remains yet to be fully elucidated [22]. The pancreas and other endoderm-derived organs develop through ‘cross-talk’ between the endoderm and the surrounding mesenchyme, which is a critical step in initiating organ specification or ‘endodermal patterning’ along the anterior–posterior axis of the foregut endoderm. Endodermal patterning is manifested by the regional expression of transcription factors in the primitive gut tube; for example, Hex1 (Hematopoietically expressed homeobox1, an early marker of anterior endoderm) and Nkx2.1 (also known as Thyroid transcription factor 1) are expressed at E8.5 in defined foregut domains along the anterior–posterior axis of the primitive gut tube, giving rise to liver and lung/thyroid respectively. Pdx1 and PTF1a are co-expressed in the foregut–midgut endoderm boundary, defining the pancreas and duodenum, whereas Cdx1, and Cdx4 (early markers of posterior endoderm) are expressed in the posterior midgut and hindgut domains that will give rise to the small and large intestines. Thus, various domains of the primitive gut tube are specified [23, 24].
Mesenchyme-induced endodermal patterning is necessary before the initiation of organogenesis. When the pancreatic mesenchyme is removed from the pancreatic epithelium in explant cultures, it results in disrupted pancreatic cell differentiation, with the endocrine lineage being favored over exocrine [9].
The primitive gut tube is divided into three domains, foregut, midgut, and hindgut regions, each of which will give rise to specialized structures [13]. This subdivision into presumptive gut tube domains is governed by different molecular markers in the gastrula stage endoderm (E7.5) [13, 25, 26]. The endoderm toward the anterior side of the embryo generates the ventral foregut, which will later give rise to the liver, lung, thyroid, and the ventral pancreas. The dorsal region of the definitive endoderm, on the other hand, contributes to the formation of the esophagus, stomach, dorsal pancreas, duodenum, and intestines. The pancreas has been found to form as a result of the actions of some key specific transcription factors and signaling pathways. Fore example, FGF4 and Wnt signaling from the posterior mesoderm are specifically inhibited in the anterior endoderm to allow foregut development. FGF signaling is required to initially determine, and then to maintain gut tube domains, as demonstrated with cultured mouse endoderm and by in vivo studies in chick embryos. FGF4 is normally expressed in the mesoderm and ectoderm adjacent to the developing midgut–hindgut endoderm, and when isolated mouse endoderm is cultured in the presence of high concentrations of FGF4, a posterior (intestinal) endoderm was induced. On the other hand, lower concentrations of FGF4 induced a more anterior (pancreas–duodenal) cell fate. Similarly in chick embryos in vivo, when treated with FGF4, Hex1 (anterior endodermal marker) expression domain was reduced, whereas CdxB (posterior endodermal marker) expression expanded anteriorly, inhibiting the development of the foregut [23, 27]. Therefore, FGF4 plays a critical role in endodermal patterning by repressing anterior (foregut) fate and promoting posterior (intestinal) endoderm fate. Another molecular pathway that has linked endodermal patterning to the initiation of pancreatic development is Wnt/β-catenin signaling, as demonstrated in frog (Xenopus) studies [28]. β-catenin repression in the anterior endoderm is specifically necessary to initiate liver and pancreas development, and to maintaining foregut identity. Conversely, forcing high β-catenin activity in the posterior endoderm promotes intestinal development and inhibits foregut development. McLin et al. [28] demonstrated that forced β-catenin expression in the anterior endoderm (where β-catenin is usually repressed) led to downregulation of Hhex, as well as other foregut markers for liver (for1), pancreas (pdx1), lung/thyroid (nkx2.1), and intestine (endocut) [25], resulting in inhibition of foregut fate, namely liver and pancreas formation. Repressing β-catenin in the posterior endoderm (future hindgut that normally expresses β-catenin) induced ectopic liver and pancreas markers (hhex,pdx1, elastase, and amylase) with subsequent ectopic liver bud initiation and pancreas development [28]. The homeobox-containing gene Hhex, is a direct target of β-catenin and is one of the earliest foregut markers [20] and is essential for normal liver and ventral pancreas development in mice [21, 29]. Hex expression was noted to have an important role in the specification and differentiation of the ventral pancreas, where Hex−/− null mutant mouse embryos lacked a ventral pancreas, and lacked liver, thyroid, and parts of the forebrain [21, 30].
Retinoic acid (RA) signaling has been implicated as an important molecule for endodermal patterning in zebrafish. RA-signaling is necessary for specification and differentiation of both liver and pancreas [31]. As with RA, BMP signaling has also been shown to have a role in endodermal patterning and in the normal development of the pancreas in zebrafish, but neither RA nor BMP affect the induction of endodermal precursors [32]. Targeted disruption of the Pdx1 gene in mice also prevented pancreatic development [33]. A critical role for Pdx1 in pancreatic initiation and patterning of foregut endoderm in mice was further demonstrated by humans with Pdx1 mutations being apancreatic [34]. Unfortunately, despite our knowledge of many molecular signals mediating cross-talk between the pancreatic mesenchyme and the epithelium, most pathways remain poorly understood.
Pancreatic Mesenchyme
Following regional specification of the foregut tube, the pancreatic epithelium becomes enveloped by the pancreatic mesenchyme. Mesenchymal factors then promote growth and differentiation of the developing pancreas, specifically inducing growth of the endocrine cell population and rapid branching morphogenesis [1]. The early stages of pancreatic development involve a lineage selection by the pancreatic epithelium between endocrine and exocrine lineages [9], which is regulated by stimuli from the pancreatic mesenchyme [1]. This interaction between pancreatic mesenchyme and epithelium is a vital process for pancreatic development. Pure pancreatic epithelium (E11) without its mesenchyme failed to develop at all, however, the epithelium grew into a fully differentiated pancreas (acinar, ductal, and endocrine structures), when cultured with its mesenchyme [9]. The pancreatic mesenchyme has a pro-exocrine effect on the epithelium through cell–cell contact, and then also a pro-endocrine effect, mediated by diffusible factors secreted from the mesenchyme [35]. Mesenchymal contact with the epithelium both enhances notch signaling (Hes1), which favors the acinar lineage, and also inhibits neurogenin 3 expression (Ngn3) leading to the suppression of endocrine differentiation [36]. The ‘default’ differentiation of the pancreatic epithelium in the absence of mesenchyme is endocrine [9]. Interestingly, culturing pure pancreatic epithelium in a basement membrane rich gel, without its mesenchyme, led to the predominant formation of ductal structures. These results suggest that the basement membrane has factors or components which are conducive to ductal development [9]. To further illustrate the importance of the mesenchyme and mesenchymal signaling in embryonic and organ development, when the normal embryonic separation that occurs between the spleen and the pancreas-associated mesenchyme does not occur in Bapx1 null mutant embryos, the dorsal pancreatic bud gets intestinalized [37]. Activin A, which is expressed in the splenic mesenchyme, is a possible mediator for this transdifferentiation since exposing pancreatic buds to activin A in an in vitro culture system, leads to intestinalization [38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree