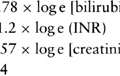Palliative Radiation Therapy
Andrea Bezjak
Peter Kirkbride
Rebecca Wong
Introduction
Palliative radiotherapy (RT) is radiation treatment administered to improve symptoms and relieve suffering. Half the patients who are diagnosed with cancer will receive RT at some point in the course of their disease, with a variety of intents—curative, radical, adjuvant, palliative, or prophylactic; these terms are defined in Table 49.1. An estimated 40–50% of all RT courses are palliative in nature (1), although that figure may underestimate palliative RT usage as it does not include high-dose “curative” treatments that are also used for tumor control or the prevention and relief of distressing symptoms rather than expectations of cure. In this chapter, we will define what is meant by “palliative RT,” review the rationale for its use, and discuss the evidence on which current palliative practice is based by answering the basic questions of “What, Why, Who, When, How, and Where.” As background, the reader may refer to the “Rules For The Practice of Radiation Therapy” (2) (Table 49.2) which are based on the ethical principles used to guide the practice of medicine.
Table 49.1 Definitions of Terms Used to Describe the Intent of Radiation Therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Table 49.2 Rules for the Practice of Radiation Therapy | ||
|---|---|---|
|
What is Palliative Radiotherapy?
Therapeutic radiation is part of the electromagnetic spectrum; it consists of high-energy photons generated from a series of complex interactions in a linear accelerator (x-rays), or directly from the nucleus of a radioactive isotope such as cobalt 60 (γ rays). Unlike “brachytherapy,” in which radioactive sources are placed directly into or close to the tumor in the patient’s body, external beam RT is delivered from an outside source to the patient’s body. Definitions of these and other commonly used terms are contained in Table 49.3. Radiation can cure many radiosensitive cancers, although high doses are required to eradicate all tumor cells. Typically 66–70 Gray (Gy) or more are delivered in daily treatments or “fractions” (often of 1.8 or 2 Gy per fraction) over 6–8 weeks. Side effects of RT are related to normal tissue injury and include both “acute” (during or shortly after completion of RT, e.g., mucositis, diarrhea) and “chronic” (months to years after RT, e.g., fibrosis). It is the normal tissue tolerances that limit the doses of RT that can be safely delivered. Therefore, when the cancer cannot be cured and the goal of treatment is symptom relief, it is logical to administer a lower dose of RT over a shorter period of time.
Table 49.3 Definitions of Common Radiotherapy Terms | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The time between RT fractions allows normal tissue cells to repair and regenerate. It also allows for previously fraction-resistant tumor cells to become more radiosensitive by becoming oxygenated or by moving to a different phase in the cell cycle. The use of smaller daily fractions (2 Gy or less) also significantly reduces the risk of late complications of RT such as fibrosis or necrosis. Higher RT doses lead to more acute effects, which are virtually unavoidable in curative treatment. In the palliative population, the same extent of acute effects would not be acceptable, as they would negatively affect quality of life over a time that may represent a substantial proportion of the patients remaining life span. As a result, in palliative RT complete tumor ablation is not the goal; symptom relief can be produced with fewer number of larger RT fractions; and the late effects which take several months or years to develop, are less relevant to a population with a short life expectancy. Consequently, palliative RT is typically administered using lower total doses (frequently 8–30 Gy), larger daily fraction sizes (3–10 Gy per fraction), and shorter total treatment times (1 day–2 weeks) than curative RT. The resultant side effects are less intense (due to lower total doses) and less prolonged (due to shorter overall treatment time). The actual effect on the tumor is greater than the same total dose administered in conventional 2-Gy fractions (as the biological dose is greater for larger fraction sizes).
Why Should We Consider Palliative Radiotherapy?
Palliative RT is widely used for a variety of oncological problems. It is effective not only for the relief of cancer-related pain and symptoms but is also well tolerated with relatively mild local side effects. The traditional indications for palliative RT are illustrated in Table 49.4. In general, palliative RT can be used to treat both primary tumors and metastases provided the lesion being irradiated is directly responsible for a symptom. The potential benefits need to be balanced against the expected side effects of RT, as well as expected responses to alternative palliative measures. If the patient’s symptoms are pharmacologically well controlled, it is not always necessary to irradiate. Then one needs to consider other clinical benefits from RT, such as a decrease in opioid requirements, and the resultant reduction in drug side effects such as constipation.
Table 49.4 Indications for the Use of Palliative Radiotherapy | |
|---|---|
|
Typically, palliative RT causes only mild and transient side effects due to the lower total RT doses commonly employed. In addition, it is usually tolerated well by all but the most infirm of patients. However, as many side effects develop post-RT, those caring for patients after RT need to be counseled accordingly.
Who Should Receive Palliative Radiotherapy?
The essential axiom of palliative RT is its use in patients who have local symptoms attributable to the presence of a malignant tumor, although patients at risk of symptom development may also benefit. There are few contraindications to palliative RT. Many patients deemed not suitable for radical treatment due to poor performance status, coexisting medical conditions, or extensive disease, may benefit from palliative RT. For example, patients with poor lung function and limited respiratory reserve unable to undergo surgery or radical RT may still benefit from low-dose thoracic RT with its lower risk of toxicity. Similarly, patients with collagen vascular diseases, who often experience exaggerated and severe reactions to radical RT, may be considered for palliative RT, although the risk-benefit ratio of the treatment needs to be thoroughly discussed.
Radiation may not be appropriate for bed-bound patients in the final stages of their illness, as they may not live long enough to experience its benefit. If a patient’s poor condition is a consequence of severe pain and/or large doses of opioid analgesia, it may be possible to achieve meaningful benefit, although symptom relief may take between 1 and 4 weeks. In such situations, the use of the “one-stop-shop” approach, where a patient can be assessed, his/her treatment planned, and treatment rendered with a single fraction all on the same visit, may be useful (3).
There is a common misapprehension that patients who have received RT cannot be re-treated in the same area. There is no doubt that if a patient has previously received radical treatment, it may be challenging or even impossible to administer a second high-dose course to the same area. However, in the palliative situation, the risk of late RT complications is less of an issue. As a result, it may be feasible to administer low-dose RT to a previously treated area. Depending upon tumor location, it is possible to repeat lose-dose treatments. Although some patients may benefit, it is not always clear whether additional RT is beneficial after failure to derive a positive response from an initial course of palliative RT. Reirradiation issues are further discussed in the section that follows.
When Should Palliative Radiotherapy Be Given?
Radiotherapeutic Emergencies
A true radiotherapeutic emergency occurs when failure to deliver treatment within a few hours or days could result in death or catastrophic irreversible damage. Severe pain, although distressing to the patient, is not an emergency, but should be treated with great urgency. As RT cannot produce immediate pain relief, pharmacologic measures are essential while RT is being organized and delivered. Table 49.5 lists indications for emergency RT in a large academic cancer center in Canada, the Princess Margaret Hospital, Toronto. In these circumstances, the patient should be seen by a radiation oncologist on the same day the lesion is diagnosed, and appropriate treatment should be started promptly.
Table 49.5 Situations Requiring the Emergency Use of Radiation Therapy, Provided No Other Treatment Is Appropriate/Lowercasea | ||
|---|---|---|
|
Although RT should be administered without delay, relief may not be immediate. The time taken for the benefits of RT to be observed depends on the situation but is rarely less than a few days. With the exception of a few very radiosensitive tissues and cells (e.g., lymphocytes), RT does not cause immediate cell death. Rather, it leads to the failure of cell division, and subsequently cell death at the next scheduled division. As a result, both the beneficial effects and the acute toxicities of RT take several days to manifest. Although not the norm, when treating bone metastases, large-fraction wide-field treatment such as half-body irradiation (HBI) occasionally produces same-day benefits.
Treatment of Symptoms
There is no threshold of symptom severity that a patient needs to cross to require palliative RT. When treating small or large volume tumors, RT is equally useful whether the disease is producing mild or severe symptoms with little difference in palliative effect, although side effects are usually greater with large volume irradiation. In some instances, as in patients with pain from multiple bone metastases, it may be more appropriate to treat with analgesics as this may give better overall relief with less side effects than RT. Additionally, some patients may become completely pain free if their analgesics are adjusted appropriately. In those circumstances, it may be possible to defer RT.
Prevention of Symptoms
In a situation where a patient has an incurable cancer that is asymptomatic, treatment may be deferred until symptoms arise, although this should be discussed with the patient. A randomized clinical trial by Falk et al. (4), supports this approach in the management of lung cancer. Two hundred thirty patients with incurable lung cancer and no symptoms requiring immediate RT were randomized for immediate palliative RT (17 Gy in 2 fractions) or observation with RT at the time of symptomatic progression. After a 6-month follow-up, patients who received immediate RT were no more likely to be free of chest symptoms (i.e., cough, dyspnea) or to survive than patients in the observation arm. Additionally, one third of patients in the observation arm never required RT (or chemotherapy). Therefore, the use of RT to prevent potential symptoms (of lung cancer) is not supported by evidence. Many radiation oncologists have admitted the use of palliative RT to “give hope” (5) as many patients are unhappy about a “wait-and-watch” approach and would prefer to receive active treatment. In such cases, the benefits may be minimal and patients may still be exposed to side effects. Despite the lack of evidence, prophylactic palliative RT may be used if the physician feels the patient is at high risk of developing a clinically significant morbidity such as fracture, cord compression, airway obstruction, or superior vena cava compression.
Retreatment with Radiation
Patients, who have previously received RT, whether palliative or radical, may develop new symptoms related to tumor in the same anatomical area. As palliative RT may provide symptom relief, further reirradiation should be considered although questions frequently arise as to the feasibility of further RT to a previously irradiated area. Due consideration to three key factors helps address this question: what is the residual tolerance of the normal structures? How likely is the disease to respond? How do the risks and benefits of retreatment with RT compare to other symptom management options?
Normal tissue tolerance refers to the relationship between dose levels and the risk of developing significant long-term side effects. Normal tissue tolerances are unique to each anatomic structure. For example, small bowel tolerance is in the order of 45 Gy in 1.8- to 2-Gy fractions, with an expected 5% patient population risk of serious small bowel serious complications (e.g., bowel obstruction). The risk of toxicity escalates with increasing doses. Normal tissue tolerance is further affected by treatment volume and underlying medical conditions. Some normal tissue repairs occur with the passage of time although many of the changes due to high-dose RT, such as fibrosis, vascular changes, and depletion of parenchymal cells, are permanent. Therefore, previous RT doses, even if remote, need to be considered, when assessing the risk of reirradiation.
How the symptom and the disease are likely to respond to reirradiation are important factors in considering reirradiation. Palliative RT provides symptom relief by a complex and poorly understood mechanism that includes, but is not limited to, killing tumor cells, leading to a reduction in tumor volume. Patients who previously responded well to RT are expected to respond again, although possibly with a lower probability and durability of response. At the cellular level, previously irradiated areas may represent an environment with a greater proportion of resistant cancer cells, and regions of hypoxia that may lead to an attenuated response.
Finally, alternative treatment options should always be considered, so as to select the approach with the least side effects and the best likelihood of benefit. Literature describing the effectiveness of reirradiation is typically scant and confined to retrospective reviews. Patients administered reirradiation represent a minority of those initially treated. The selection criteria for reirradiation are generally not well described, making generalization difficult. Altered fractionation (lower dose per fraction) has been used in an attempt to lower the risk of late effects (6), although evidence to support its superiority is limited.
The evidence regarding reirradiation for specific clinical indications (bone metastases, brain metastases, lung cancer) is described in corresponding sections of this chapter. In all of the common indications for palliative RT, only highly specific patients are offered reirradiation. Patient selection is typically based on the feasibility of delivering a reasonable dose and volume, the predictability of acceptable risks of late toxicities and favorable performance status and life expectancy. The low risk of generally observable late effects is likely the combination of a meticulous planning technique, choice of dose fractionation, exclusion of patients at high risk of developing toxicities, and, in cases such as brain metastases, the shorter life expectancy that limits the manifestation of late effects. Despite these limitations, reirradiation has a definitive role in providing symptom relief in selected patients and should be considered where appropriate.
“Opportunity Costs”
Although individual RT treatments often take only 10–20 minutes to deliver, time spent traveling and waiting for treatment can add up to several hours. A protracted course of RT, especially for patients living some distance from the treatment center, may occupy most of their waking hours. One needs to remember that “time is precious when life is short” (2). For each patient, the benefits of treatment need to be compared with the cost of “lost opportunities” for them to spend their remaining days as they choose (7). In many situations, it appears likely that large single fractions are as efficacious as longer courses (see next section). Even if they were not, it may still be appropriate in patients when the “opportunity cost” of a 2-week treatment would be extremely high. Reduction in palliative benefit would be minimal at most, and concerns about the duration of the effect of single treatments are probably not relevant in patients with extremely limited prognosis.
How Much Radiation Should Be Given?
Fractionation schedules for palliative RT have evolved based on clinical experiences rather than exact science. Experimental treatment models have been developed and continue to be refined, increasing the understanding of total dose effect, fraction size, and treatment time on radical course RT outcomes. Until recently, minimal literature has been published on how these variables influence noncurative RT. Many palliative fractionation regimes exist due to physician personal preference and are often based on a variety of influences such
as departmental policies, machine availability, training, and concern about acute and late side effects (8).
as departmental policies, machine availability, training, and concern about acute and late side effects (8).
Initially, schedules were developed pragmatically by physicians who realized the following:
A full radical dose of radiation was inappropriate in a patient who could not be cured
Symptoms could be significantly relieved by smaller total doses
Shorter treatment times were more convenient for patients and their families
Most of the basis of palliative RT can be traced to recommendations from 1948 that “a useful palliative dosage was one half or better still two thirds of a tumor lethal dose” (9). Within the last 10–15 years, there have been more controlled clinical trials and increased research comparing different palliative prescriptions for various clinical scenarios.
Where in the Body Can Palliative Radiotherapy Be Effective?
The following sections deal with the sites most commonly irradiated palliatively. Non–small cell lung cancer (NSCLC) is the primary tumor most commonly treated with palliative intent. Bone and brain are the most common sites of metastatic disease for most cancers and occupy a large workload percentage of a typical RT department. RT can also be useful to palliate pelvic disease, lymph node metastases, and spinal cord compression. Although liver metastases are common, they are less often palliated with RT because of the toxicity of irradiating large volumes of hepatic tissue and lack of palliative benefit in all but the most radio-responsive tumors. Recently, there have been more studies documenting the role of radical RT doses in good prognosis patients for solitary or few liver metastases, in which RT can produce local control.
Lung Cancer
Lung cancer is the most common cancer in North America and the most common cause of cancer deaths in both men and women. Most patients either present with stage IV (metastatic and incurable) disease or develops metastases some time after the initial diagnosis. Although advances have been made with palliative chemotherapy, palliative RT continues to play an important role in the management of intrathoracic (and extrathoracic) disease, including endobronchial or extrinsic lesions causing atelectasis, postobstructive pneumonia, shortness of breath, cough, hemoptysis, pain, and large airway obstruction. Not surprisingly, RT is not effective in relieving shortness of breath resulting from widespread or parenchymal disease, pleural effusion, or lymphangitic carcinomatosis. Symptoms due to nerve compression (e.g., superior sulcus/Pancoast tumors and intercostal neuropathic pain) are more difficult to palliate and may require high doses of RT aimed at tumor eradication.
The relevant issues in assessing palliation in patients with lung cancer are the following:
Symptom relief, including the rapid onset of symptom relief; the degree of relief (partial or complete improvement), and the duration of symptom relief
Whether the patient has only one symptom or a conglomerate of symptoms (e.g., hemoptysis is easier to palliate than dyspnea)
The toxicity of treatment
Any change in the patient’s performance status
Obviously the interplay between these issues is complex, and most research studies only attempt to measure some of these variables.
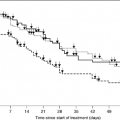
Numerous palliative RT trials have been performed; the more recent ones are highlighted in Table 49.6 (10, 11, 12, 13, 14). Most of the dose-fractionation schedules demonstrated considerable symptom palliation. Many studies have suggested that less protracted radiation protocols (including 17 Gy in 2 fractions 1 week apart and 10 Gy in a single fraction) (11) provide better palliation (i.e., equivalent symptom control with less toxicity and less burden to the patient and family). In a recent Norwegian study comparing short, intermediate and long schedules of 17 Gy per 2 fractions, 42 Gy per 15 fractions and 50 Gy per 25 fractions, there was no difference in quality of life, symptom response or survival among the three study arms (15). In the shorter treatment protocols, approximately 80–85% of patients reported improvement in hemoptysis while 60% reported improvement in cough and two thirds reported pain improvement. On an average, symptom relief lasted for at least 50% or more of the patient’s survival time. A few studies, on the other hand, have documented a longer duration of symptom control with the more protracted fractionation: in the Dutch trial by Kramer et al. (16), palliation in the 10 × 3 Gy arm was more prolonged than in 2 × 8 Gy. Similarly, a recently published study by Erridge et al. (17) demonstrated superior symptom control with a more protracted course, consisting of 30 Gy per 10 fractions as compared to 10 Gy single fraction. It should be noted, however, that defining and analyzing symptom palliation in lung cancer is complex, as the effectiveness of palliative radiation may be related to initial symptom severity (18). Timing of the assessment is also important (19) as a standard definition of palliation is lacking. These methodological concerns may be contributing factors to some of the disparities seen in various studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree