Palliative Orthopaedic Surgery
Juan Santiago-Palma
Juan C. Jimenez
Metastatic bone disease is the most common malignant bone lesion in adults. Seventy to 80% of all patients with cancer are likely to develop bone metastases (1) and approximately 9% will sustain a pathologic fracture (2). For many, a pathologic fracture is an indication of end-stage disease. It is estimated that only half these patients will survive beyond 1 year (3).
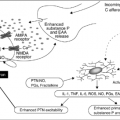
Bone metastases cause significant pain. Metastatic lesions produce inflammatory and osteolytic factors, which can activate nociceptors (4, 5). and recruit osteoclasts (6, 7). Patients experience pain as the tumor grows and increased tissue edema and intraosseous pressure develop. Osteoclastic activity erodes the cortex of the bone, which can eventually result in a pathologic fracture (6, 7).
Over the past decade attention has been directed toward improving care for patients with bone metastases. Advancement in radiotherapy, surgical techniques, and the development of new drugs has improved the quality of life of patients with cancer and bone disease. Despite these advances, the management of painful bone metastases remains palliative.
In patients with metastatic bone disease the general objective of treatment shifts from curing the patient to providing symptom control and improving quality of life.
The cornerstone of treating symptomatic bone metastases is a combination of analgesics, external beam radiation therapy (EBRT), and bisphosphonates. Surgery, nerve blocks, chemotherapy, steroids, and hormones must be considered as adjunct therapies. In most patients, a combination of systemic modalities is required to keep the patient free of pain for extended periods of time. The key is to choose from different treatments to optimize pain, decrease treatment side effects, improve function, and quality of life.
Evaluation
Pain is the most common symptom of bone metastases. Patients with skeletal disease present with pain over 75% of the time (8). Bone pain is the result of mechanical stress on the weakened bone, destruction of the bone, microfractures, periosteal distension, nerve compression, and tumor pressure on adjacent tissues. In addition several chemical factors, which mediate osteolytic, bone resorption, such as prostaglandins, bradykinin, substance P, interleukin-1 (IL-1), IL-6, and tumor necrosis factor activate the pain receptors of the periosteum (9).
Metastatic disease should be considered in any patient with limb or spine pain and a known history of cancer. Prostate and breast cancer are the most common tumors that cause bony metastases. Myeloma, lymphoma, and cancer of the lung and kidney are the most common malignancies with a lesion of bone as their first presentation (10).
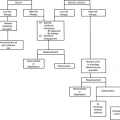
The standard approach to evaluate a patient with metastatic bone disease is to follow an algorithm of clinical examination, laboratory tests, and imaging studies. A comprehensive evaluation must be made to determine the possibility of an impending pathologic fracture or neurologic compromise such as spinal cord compression.
Bone pain produces a well-localized dull ache that increases with weight bearing. Pain is usually aggravated with moving, standing, and walking. There is often focal tenderness. Bone pain is often associated with other pain syndromes. Metastases to the vertebrae may cause spinal cord compression, nerve root compression, or cauda equina syndrome. Metastases to the base of the skull can impinge on cranial nerves.
Initial diagnostic workup usually includes x-ray of the affected limb or spine. Plain radiographs are useful to assess for bony changes and fractures and can determine if the lesion is osteolytic or osteoblastic. Purely lytic lesions are prone to fracture, whereas purely blastic lesions seldom fracture (11). Bone metastases in patients with breast cancer and multiple myeloma are predominantly osteolytic in nature. By contrast, prostate bone lesions are mixed, containing both osteolytic and osteoblastic elements (12). Osteolytic lesions may not be apparent for several months, because, 30–75% of normal bone mineral content must be lost before the lesion becomes apparent on x-rays (13, 14).
Bone scanning with technetium-99 is the most common diagnostic test used to detect bone metastasis (15, 16). Bone scan detects increases in osteoblastic activity and skeletal vascularity (17, 18). Sensitivity and specificity rates of technetium bone scanning range from 62–100% and specificity from 78–100% (19, 20, 21, 22, 23). Bone scan is considered sensitive for detecting osteolytic or osteoblastic bone metastases (24). False-negative findings can occasionally occur when osteolytic metastases grow rapidly, when bone turnover is slow, or when the tumor site is avascular (24).
Computed tomography (CT) scan offers excellent detail of the bone and bone marrow. CT scan is useful to distinguish structures of different densities. The sensitivity of CT scan for the diagnosis of bone metastases ranges from 71–100% (25, 26).
Magnetic resonance imaging (MRI) provides detailed imaging of the bone and bone marrow. The diagnostic sensitivity of skeletal MRI ranges from 82–100%, and its specificity ranges from 73–100% (27, 28, 29, 30). Bone marrow lesions are better visualized in MRI. MRI also has better resolution than CT scan for
soft tissue and neurovascular structures. MRI is less accurate than CT scan to detect destruction of bone (17, 31).
soft tissue and neurovascular structures. MRI is less accurate than CT scan to detect destruction of bone (17, 31).
Positron emission tomography (PET) visualizes the uptake of positron-emitting radiopharmaceuticals by tissues. PET is used for whole-body scanning to detect metastases in either soft tissue or bone. Fluorodeoxyglucose (FDG) PET measures glucose metabolism in many types of cancer and can be useful for distinguishing benign from malignant bone lesions (32, 33, 34). Estimates of the sensitivity of FDG PET for detecting bone metastasis range from 62–100%, and specificity from 96–100% (35, 36, 37). The disadvantages of PET are its high cost and its relative lack of availability Single photon emission computerized tomography (SPECT) uses the same radionuclide as bone scans. Like CT scan, SPECT uses a rotating camera to create tridimensional cross-sectional images. SPECT is useful to evaluate areas of increased uptake seen in bone scans due to its better resolution (38, 39). Its sensitivity for the diagnosis of bone metastases is 87–92%, and its specificity is 91–93% (40, 41). SPECT is useful to evaluate areas that are extensively surrounded by soft tissue such as the thoracolumbar spine and pelvis. In the spine SPECT useful for distinguishing benign from malignant lesions (42).
Surgical Treatment
The aim of surgery in metastatic bone disease is to stabilize the weakened bone and to eliminate the risk of pathologic fracture. Surgical treatment of a bone metastasis should be preceded by careful evaluation of the patient’s needs, wishes, and prognosis. The goal of surgical treatment is to reduce pain, maintain function, and improve quality of life. A pathologic fracture is an obvious indication for surgery. Indications for surgical treatment without a fracture are less clear and must take into consideration the prognosis of the patient, failure of more conservative therapies and patients desire to proceed with surgery.
Studies indicate that the 1-year survival of patients with skeletal metastases who undergo surgical treatment is poor. Most studies have shown a 1-year survival of 0.30–0.54 (43, 44, 45). Various clinical features may help assess the survival in patients with metastatic bone disease. Patients with multiple myeloma, lymphoma, kidney and breast cancer have a better prognosis (38, 39, 40). and patients who suffer from lung cancer have a poorer prognosis. Complete pathologic fractures, and known visceral metastases are negative prognostic factors (41, 42, 43). Patients with poor Karnofsky scores, weight loss, and hemoglobin <7 mg per dL have a shorter survival (43, 46, 47).
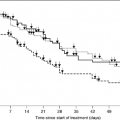
There is no evidence that the surgical fixation of bone metastases influences long-term survival. Survival of patients who undergo surgery for bone complications is similar to that for patients with cancer who are treated by radiotherapy for bone pain. In a large randomized trial of radiotherapy for bone pain, the median survival was 6 months as compared with 6 months for patients who underwent surgery (45, 48).
The decision to proceed with surgery in patients who are at risk of fracture is a complicated one. Although the avoidance of pathologic fracture is desirable, the principle of prophylactic surgery remains controversial. An attempt should be made to prevent fracture, by radiotherapy and the administration of bisphosphonates. Some studies have suggested that prophylactic fixation can result in decreased morbidity, shorter hospital stays, easier rehabilitation, and more pain relief when compared to fixation of pathologic fractures (49). However, survival curves have shown that if the bone does not fracture within 3 months, the chance of death occurring is 35%. Furthermore, pathologic fractures are relatively rare. In two trials for bone pain the rates for a long bone fracture were only 5 and 10% respectively (48, 50). In a population-based study of patients with cancer the risk of a long bone fracture was 12% (51).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree