PALLIATIVE ENDOSCOPIC PROCEDURES
Interventional endoscopy has evolved into a major instrument for treating patients who suffer from gastrointestinal tumors and to relieve severe ailments such as dysphagia, ileus, intestinal bleeding, icterus, sepsis, and pain. Mainstays in the development of the endoscopic armamentarium were the development of endoscopy-based antitumor therapies, the advances in the construction of self-expanding metal stents (SEMSs), the rapid evolution of interventional endoscopic ultrasound techniques, and the increasing use of combining percutaneous and luminal (endoscopic) treatment approaches (“Rendez-vous” technique).
An important endoscopic task is palliative care of intestinal obstruction in advanced abdominal or pelvic tumors. There has been a significant shift from noncurative surgical management to endoscopic treatment in intestinal obstruction in the last few years, and a detailed overview on the indications, endoscopic technique, and outcome of the management of intestinal obstruction is therefore provided. Specific endoscopic treatment approaches are available to stop bleeding from intestinal tumors, to treat cancer with local ablation techniques (e.g., bile duct cancer and photodynamic therapy [PDT]), to manage biliary obstruction, and to alleviate pain in pancreatic cancer by endoscopic ultrasonography (EUS)-guided lysis of the celiac plexus. These subjects are therefore separately discussed. Moreover, there is a concise overview given on enteral nutrition support and on treating infectious complications by endoscopic means.
ENDOSCOPIC MANAGEMENT OF INTESTINAL OBSTRUCTION
Intestinal obstruction is a severe complication of intra-abdominal and pelvic malignancies. The incidence of bowel occlusion is not exactly known but is frequent in advanced tumor stages and occurs in 6% to about 50% of patients with ovarian cancer (
1,
2,
3) and in 10% to about 30% of patients with colorectal cancer (
1). Related symptoms significantly compromise the quality of life of the patient, and clinical management may be a challenge. Etiology of bowel obstruction is heterogenous, though, and may be benign in up to 40% of cases. Extrinsic (abdominal mass, intestinal or peritoneal carcinomatosis, fibrosis, adhesions, and radiation enteritis) and intrinsic lesions (malignant polyp and fibrosis) are involved, and mechanical obstruction must be discerned from paralytic ileus (
Table 21.1). Risk factors for intestinal obstruction are advanced tumor stage, residual primary tumor, and presence of intestinal carcinomatosis. Symptoms at primary presentation are indicative of the site of the obstruction: dysphagia is caused by oropharyngeal or esophageal lesions or may be of functional origin, whereas mechanical ileus is the sequel of intestinal obstruction and results in colicky pain, abdominal distension, vomiting, dehydration, and, potentially, hypovolemic shock. Immediate diagnostic clarification is mandatory to avoid aggravation of symptoms. Plain abdominal radiography may demonstrate fluid levels. Water-soluble oral contrast can help define the extension and the site of the obstruction. Abdominal computed tomography is useful to locate the exact site of obstruction and to further characterize the reason for it. Endoscopy helps to locate a stenosis and biopsies can determine its etiology.
Antiemetics, steroids, and octreotide are used to relieve symptoms, and metoclopramide is helpful for functional obstruction (
1,
4). A nasogastric tube is a temporary measure in patients unresponsive to medical treatment, whereas a percutaneous gastrostomy may be placed for permanent gastric decompression. Surgical interventions with bypass procedures and/or segmental bowel resection are associated with significant morbidity, a complication rate of 37% to 64%, and in-hospital mortality of 10% to 30% or greater (
2,
3,
5,
6,
7) (
Table 21.2). The major underlying value of an operative intervention may be primarily to identify a benign etiology of obstruction in selected cases and only secondarily to alleviate the consequences of carcinomatosis (
1). Candidates for surgery must present with a good clinical performance status to achieve acceptable outcomes (
9).
Recent advances in SEMS technology have significantly widened their indications for patients with intestinal obstructions. Nitinol, a melt of nickel and titanium, is the primary metal used for SEMS construction. Shape memory, elasticity, maximum expansion force at body temperature, and application with relatively small delivery devices that allow for TTS (“through-the-scope”) insertion are characteristics that favor the use of this material. Other substances presently in use include stainless steel and plastics. The covering of SEMS may be made of various materials, including silicone, polyurethane, and expanded polytetrafluoroethylene. The cover may extend over the entire length of the stent (fully covered SEMS) or leave small areas at the ends uncovered (partly covered SEMS).
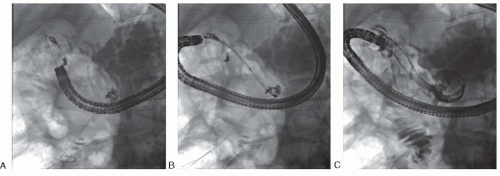
Sites of obstruction can often be bridged in the upper gastrointestinal tract, the proximal small bowel, and the colon with SEMS, though there are some practical caveats. Importantly, combination of OTW (“over-the-wire”) and TTS techniques minimizes the risk of intraprocedural perforation
in the duodenum, small bowel, and colon. Fluoroscopy is essential to provide secure access beyond the stenosis in case it cannot be traversed endoscopically. In this situation, carefully placing a hydrophilic guidewire through a catheter after contrast injection enables the visualization of the stenosis. The guidewire will transverse the tumor in almost all cases, even if small-caliber endoscopes are not able to pass the obstruction. Moreover, the air column seen with fluoroscopy on the opposite side of the tumor (stenosis) indicates the direction in which to advance the guidewire (
Fig. 21.1).
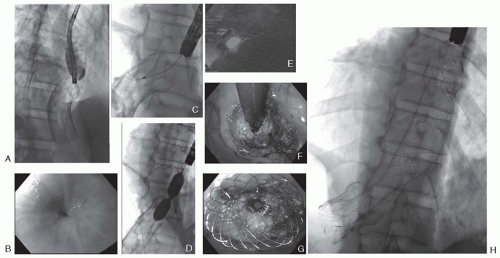
Stent retrieval is rarely necessary in malignant obstruction. Removal may be accomplished with oral or aboral retraction, stent turnover in the stomach, extraction by covering of an overtube, untying the stent by extracting the thread (in case of knotted SEMS from one thread of metal), inversion of the stent (invert from distal to proximal), excavating or trimming the stent by welding it or destroying it in corpora. While fully covered SEMSs are often easily extracted, removal of noncovered stents is difficult particularly after the elapse of a long time from stent placement (more than several months) or if tumor ingrowth, scarring or granulation tissue provoke stent closure at the stent endings. Granulation tissues can sometimes be removed by inserting a second, fully covered stent within the partially or noncovered stent, for example, in esophageal or in biliary SEMS (
10) (
Fig. 21.2).
Patients with multiple consecutive strictures—for example, in peritoneal carcinomatosis—may be poor candidates for SEMS placement. Moreover, patients with a short life expectancy, for example, less than 4 weeks, should probably not be considered as candidates. Careful patient selection is of high importance. Endoscopic tumor destruction (argon plasma coagulation [APC], ethanol injection, Nd-YAG laser, and mechanical debulking) is infrequently performed because repeated sessions are usually necessary with a negative effect
on quality of life because of repeated hospitalizations for a patient with limited life expectancy. Moreover, risk of perforation with these procedures is at least 10% (
11), and these are, therefore, reserved for patients with obstructions not amenable to other therapies.
Esophageal Obstruction
The indications for endoscopic therapy for advanced esophageal or mediastinal tumor are relief of dysphagia, maintenance of nutrition, or sealing of a tracheo-esophageal fistula. Immediate success after SEMS placement for these symptoms is as high as 95%, but long-term stent dysfunction occurs in about 20% to 30% of patients (
12,
13). At present, noncovered, partially covered, and fully covered SEMSs are all in use (
14). Covered or partially covered SEMS may be preferred over noncovered stents because tumor ingrowth is prevented by the stent cover, but dislocation of these stents may be somewhat more frequent (
15). When compared to SEMSs, self-expandable plastic stents (SEPSs) provide equal improvement of dysphagia due to tumor stenosis, but stent migration is more common with SEPS (
16).
The use of a covered SEMS for the treatment of esophageo-bronchial fistulae improves survival. Palliation of malignant fistula is accomplished with an esophageal stent implantation or with parallel (esophagus plus airway) SEMS insertions. In some cases, dual stents provide improved palliation and safety. Particular attention must be paid to potential tracheal compression secondary to esophageal stents. The stent may, depending on the model, have a pronounced radial expansion force and thereby compress the surrounding tissue, including the trachea (
Table 21.3). Additional endoscopic treatment options to relieve malignant dysphagia are local tumor ablation using APC, high dose rate brachytherapy, PDT, and a combination of these therapies (
17) or in combination with chemoradiation (
18).
Gastric Outlet Obstruction
Palliation of malignant gastroduodenal obstruction is as successful with SEMS placement as with gastroenterostomy. While long-term success might favor gastroenterostomy, complication rates are lower with SEMS placement (
19,
20). Gastroenterostomy may be preferred in some patients with a longer anticipated survival and performed during abdominal exploration for potentially curative resection. SEMS is preferred for patients with poor performance status and if curative surgery is not an option at diagnosis (
21). The technical success rate of SEMS in this setting is higher than 90% and the clinical success rate is at least 75% in most studies (
22). Device-related adverse events include stent occlusion or malfunction in 9% of patients and perforation in 5% (
23). Solid food intake can generally be resumed in more than 50% of patients, and 75% benefited from significant improvement in nutritional status in a multicenter study. The shorter time from stent implantation to initiation of oral intake, low morbidity, and a brief hospital stay favor SEMS over surgical gastroenterostomy (
24). The implanted stent should be non-shortening, with maximum flexibility and sufficient radial expansion accompanied by minor axial straightening force (
22).
Duodenal tumor as a cause of stenosis has a high frequency of concomitant biliary obstruction, for example, in patients with cancer of the pancreatic head. In these cases, insertion of a biliary SEMS together with a duodenal SEMS is often most appropriate. The optimal procedure depends on the timing and the sequence of occurrence of the biliary and duodenal stenosis. In most cases, biliary obstruction precedes duodenal occlusion and a biliary SEMS is inserted first to accomplish biliary drainage. Only if a duodenal obstruction occurs some time later is a duodenal stent needed. In this case, the duodenal stent may come close to the biliary SEMS and it may need to be trimmed if the tumor involves the complete descending duodenum (D2) and a trans-papillary stent is envisaged. When biliary and duodenal obstruction occur simultaneously, biliary SEMS insertion and immediate duodenal stent placement is preferred. With an exclusive endoscopic approach, the duodenoscope is placed in the descending duodenum opposite the papilla after complete deployment of the duodenal stent. Now, the mesh of the duodenal SEMS is manipulated at the site of the major papilla (by means of APC or with dilation catheters) and biliary cannulation is attempted to insert the biliary SEMS. This technique is likewise useful in a third, less frequent scenario, when a biliary stenosis develops sometime after duodenal stenosis.
If an endoscopic biliary access is not obtained, a percutaneous SEMS insertion is needed, sometimes combined with endoscopic visualization or the Rendez-vous technique to adjust the exact placement at the level of the duodenal wall. The combination of endoscopic and percutaneous procedures offers a high potential for success, even in technically challenging situations.
Small Bowel Obstruction
New endoscopes and balloon enteroscopy technique enable ordinary endoscopic access to distal portions of the small bowel. TTS placement is limited, though, by the small working channel of balloon enteroscopes (2.8 or 2.2 mm). OTW placement of an SEMS in the distal small intestine is technically feasible but cumbersome and maybe associated with increased risk of perforation. Most importantly, though, it must be clarified that if the patient’s symptoms are due to multiple consecutive stenosis of the small bowel, as with
peritoneal carcinomatosis, this can rarely be helped with endoscopic stenting. Percutaneous gastrostomy tube placement for decompression and drainage of gastric and enteral secretions is often the best option for these patients.
Colonic Obstruction
Endoscopic palliation of advanced stage colon cancer has been performed for over 20 years. It has found a widened application in the last few years with the development of new SEMS (
Table 21.4). There are two clinical settings in which SEMS placement is appropriate. The first is for palliation, that is, diffuse metastasis or advanced comorbidity forecloses curative surgical resection of the tumor. In this situation, endoscopic SEMS is equally successful in the short term and long term as palliative surgery, if failure of stent patency is re-treated endoscopically with stent-in-stent placement (
37,
38). Moreover, SEMS insertion seems equally effective and less costly than surgery in patients with metastatic colon cancer (
42). In the second setting, potentially curative resection of the tumor is envisaged in a patient with acute colonic obstruction. In this setting, “bridge-to-surgery” by inserting SEMS and providing relief of the obstruction may avoid colostomy and facilitate a safe single-stage surgery (
43,
44) (
Table 21.5). Successful treatment of the bowel obstruction by inserting an SEMS avoids emergency surgery and significantly reduces the hospital stay (
31). This may be particularly important for elderly patients; the mortality with initial SEMS insertion is significantly lower (3%) than with emergency resection (19%) in patients with acute colon obstruction from tumor (
50).
With some SEMS models, complications have been reported with stent placement in the right side of the colon or in the colonic flexures (
33), but improvements in the stent design have reduced the complication rate (
27). Minimal axial straightening of the stent, in combination with sufficient radial expansion force, seems essential for the optimal risk-benefit balance of SEMS insertion for colorectal cancer. Ideally, the stent should align exactly with the configuration of the organ, have no tendency to migrate, and prevent ingrowth.
There are two meta-analyses, from 2004 and 2002, that review the results of endoscopic palliation of colorectal cancer by SEMS placement. Technical success was reported to be 94% (
n = 1198) or 92% (
n = 598) for SEMS insertion for palliation. The use of SEMS placement as a “bridge-to-surgery” was found to have a clinical success rate of 85% to 91%. Perforations occurred in 3.7% or 4% of cases and reported procedure-related mortality was 0.58% and 1%, respectively (
43,
58). The management of extrinsic blockage of the colon with SEMS is less frequently successful and might be as low as 20% (
30,
39). Chemotherapy with bevacizumab in colorectal cancer patients with SEMS in situ has been suggested to increase the risk of perforation (
28), though this has not been confirmed in other studies. Low rectal obstruction is difficult to treat with SEMS placement, and debulking procedures using APC, laser, or snare resection may be utilized but are rarely necessary when radiochemotherapy is available (
Fig. 21.3).