Conditions for which curative or life-prolonging treatment is possible but may fail, such as advanced or progressive malignancy or malignancy with a poor prognosis, or complex and severe congenital or acquired heart disease.
Conditions requiring long periods of intensive treatment aimed at prolonging quality of life such as human immunodeficiency virus, cystic fibrosis, severe gastrointestinal disorders, or malformations such as gastroschisis, severe epidermolysis bullosa, severe immunodeficiencies, renal failure when dialysis and/or transplantation are not available or indicated, chronic or severe respiratory failure, or muscular dystrophy.
Progressive conditions in which treatment is exclusively palliative from diagnosis such as mucopolysaccharidoses or other storage disorders, progressive metabolic disorders, certain chromosomal abnormalities such as Trisomy 13 or Trisomy 18, or severe forms of osteogenesis imperfecta.
Conditions with severe, non-progressive disability, causing extreme vulnerability to health complications such as severe cerebral palsy, extreme prematurity, severe neurologic impairments due to illness or injury, or severe brain malformations. Given the uncertainty of prognosis, many children with these conditions will not fit eligibility criteria for hospice care in the United States, which generally requires a life expectancy of 6 months or less (11).
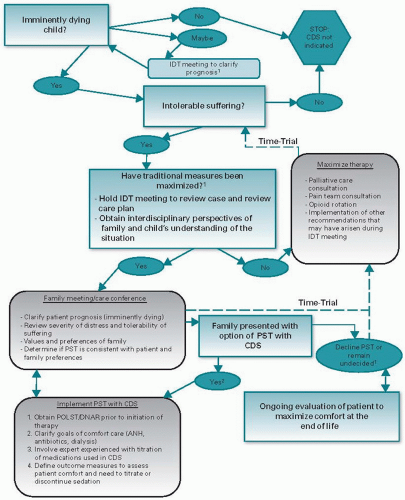
source of meaning (12). Suffering is a profoundly personal experience and is endurable, and at times even fulfilling, when it becomes meaningful (13). Despite best efforts, children living with chronic, life-threatening, and terminal illnesses experience substantial suffering. The extent, as well type, of suffering has broad-ranging implications in the child’s life as a whole and in the family as a functional unit. Understanding the illness experience of patient and families is essential. Parents who recognize that their children have a poor prognosis and believe that their children are suffering from the illness and its treatment are more likely to put greater value on comfort and quality of life as they make care decisions.
All children suffering from chronic, life-threatening, and terminal illnesses are eligible.
Care is patient and family centered and is based on continued healing relationships.
Care focuses on attending to suffering and enhancing quality of life for the child and family.
Care is provided for the child as a unique individual and the family as a functional unit.
Care is incorporated into the mainstream of medical care regardless of the curative intent of therapy.
Care is not directed at shortening life.
Care is coordinated and continuous throughout the illness trajectory and across all settings in which the child receives services.
Care is goal directed and consistent with the beliefs and values of the child and his or her caregivers.
Care is interdisciplinary and addresses all levels of the patient’s and family’s illness experience.
Advocacy for participation of the child and caregivers in decision making is paramount.
Facilitation and documentation of communication are critical tasks of the team.
Respite care and support are essential for families and caregivers.
Bereavement care should be provided for surviving family members including parents and siblings, for as long as needed.
Prognosis for short-term survival and a “Do Not Resuscitate” order are not required for eligibility.
if withdrawal leads to the patient’s natural death (such as removal of a ventilator) (33). In making these difficult decisions the right to self-determination, or autonomy, allows individuals and families to make risk-benefit determinations specific to their personal experiences and value systems. The discontinuation of non-beneficial interventions is within the scope of parental decision-making authority and should not be viewed as inconsistent with a child’s best interests. Clinicians should seek to override family wishes only when those wishes clearly conflict with the best interests of a child. Clinical ethics consultation is always advisable in these cases.
prevention, and treatment of medication side effects (65,66). Depending on the etiology of the pain being treated, there are many nonopiate adjunct therapies available. Table 63.2 lists some of the more commonly used medications and their indications. For children, in particular, choose the simplest, most effective, least painful route which for most will be oral or sublingual or for those with central venous access, parenteral. Intramuscular injections should be avoided if possible. Rectal administration is possible, and many medications, including nonopiates and opiates as well as medication for nonpain symptoms, can be absorbed rectally. Subcutaneous infusion, popular in adult hospice and palliative medicine, is possible in children but may be inadvisable for needlephobic children. Transdermal and inhalational routes may also be used in children, but little pharmacokinetic data are available regarding their use; anecdotally, absorption and/or metabolism of transdermal fentanyl may be faster in children, requiring patch changes every 2 days if breakthrough pain occurs. Promotion of good psychosocial and spiritual care must be partnered with appropriate pharmacology (67). Children should be given choices as often as possible. Behavioral methods, such as deep breathing (blowing bubbles), progressive relaxation, and biofeedback, have a role in pain management for children. Physical methods, such as
touch therapies, including massage, transcutaneous electrical nerve stimulation, physical therapy, heat/cold, and acupuncture and/or acupressure, for example, are helpful adjuncts. Cognitive modalities, including distraction, music, art, play, imagery, and hypnosis, are also effective in children. Studies have demonstrated efficacy of many of these modalities alone or in combination with pharmacologic therapies (68,69).
TABLE 63.1 Opioid and nonopioid analgesics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
with end-stage dyspnea (77). There are no studies to support the use of supplemental oxygen in children at the end of life although practical experience suggests that this treatment may have additional psychological benefits for the parents of the sick child.
TABLE 63.2 Adjuvant drugs useful in pediatric pain management | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree