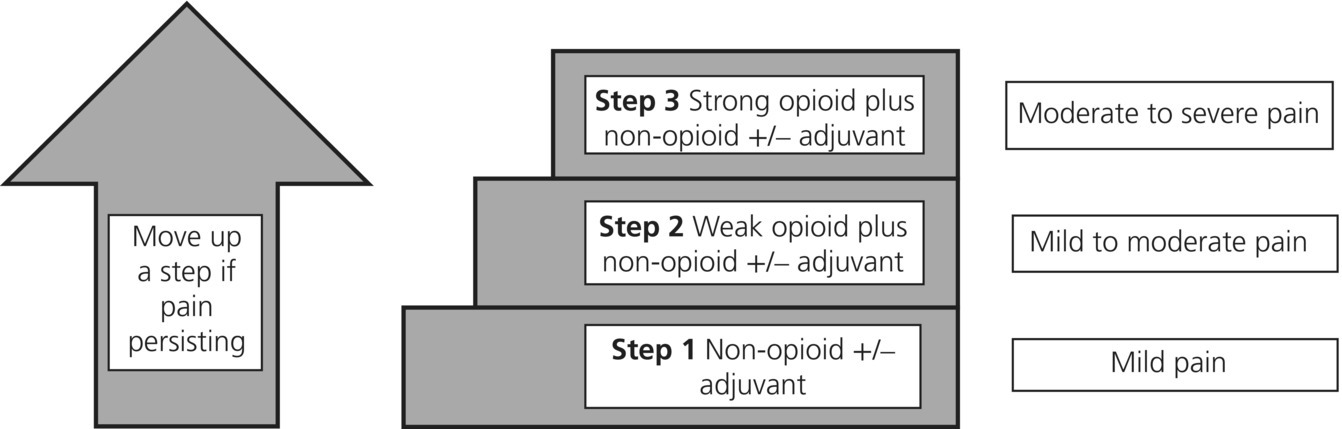
Chapter 8 Karen Neoh St Gemma’s Academic Unit of Palliative Care, UK Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. The majority of patients with cancer experience pain; therefore clinicians must understand the impact pain can have on patients and manage symptoms quickly and effectively. Table 8.1 ‘SOCRATES’: a common mnemonic acronym for assessing pain. Figure 8.1 Based on the World Health Organisation (WHO). Adapted with permission, from the World Health Organisation, WHO’s Pain Ladder for Adults 2013. Available from: http://www.who.int/cancer/palliative/painladder/en/ (accessed 1 January 2014). Bone pain can be managed with radiotherapy, bisphosphonates and NSAIDs (see section on NSAIDs above). Following treatment with bisphosphonates or radiotherapy pain may improve dramatically, sometimes allowing a reduction in the doses of regular analgesia. In 2012 the National Institute for Health and Care Excellence (NICE) published ‘Safe and effective prescribing of strong opioids in palliative care of adults’ based on systematic reviews of the best available evidence. The recommendations are summarised below:
Palliative care and pain management
Pain management
Overview of pain management
Assessment
S
Site
Where is the pain, or the maximal site of the pain?
O
Onset
When did the pain start, was it sudden or gradual? Is the pain progressive or regressive?
C
Character
What is the pain like? An ache? Stabbing? Sharp?
R
Radiation
Does the pain radiate anywhere?
A
Associations
Any other signs or symptoms associated with the pain such as dyspnoea or nausea.
T
Time course
Does the pain follow any pattern?
E
Exacerbating/relieving factors
Does anything change the pain?
S
Severity
How bad is the pain? 1–10 with 10 representing the most severe pain.
Choice of analgesic
Renal and liver failure
Bone pain
Radiotherapy
Bisphosphonates
Opioid prescribing in palliative care
General principles of opioid prescribing
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree