Fig. 9.1
Pain assessment tools. (a) FLACC behavioral pain assessment scale. (b) Wong-Baker faces pain scale. (c) Visual analog scale (VAS)
The perception of pain is directly related to a child’s age and developmental level (Gaffney and Dunne 1986). Pain is identifiable by children at 18 months of age, and children can identify where they “hurt” by 4 years. In the past it was incorrectly presumed that infants do not feel as much pain as adults. Although infants’ pain pathways are immature, they are fully able to transmit impulses (Fitzgerald 2000). Generally children <6 years need help describing their pain. Children >6 years can describe pain, and those between the ages of 7 to 11 often find themselves responsible for it or may recognize pain as a form of punishment (Esteve and Marquina-Aponte 2012). Adolescents are able to understand the multiple layers to pain consisting of psychological and physical causes. Adolescents often can be taught coping mechanisms due to this complex and abstract view of pain.
A complete assessment should include a thorough clinical examination. Facial expression should be noted in infancy as a behavior signaling pain (Izard et al. 1987). Pain in this age group, as well as in children with developmental delay, is typified by facial grimacing, quivering of the chin, grunting and clenching of the jaw. Other signs of pain in infants include kicking, arching, high-pitched crying and inability to be consoled (Krechel and Bildner 1995; Merkel et al. 1997). Physiological changes in heart and respiratory rate, blood pressure, and oxygen saturation can be evoked by pain but often do not indicate the level of pain in clinical encounters (Sweet and McGrath 1998).
9.3 Types of Pain
Two primary types of pain, nociceptive and neuropathic, have been described. Identification of the type of pain can be made by history and physical exam along with the input of imaging modalities. Delineating which type of pain a patient is experiencing is imperative as it guides therapy and may aid in determining an underlying etiology.
9.3.1 Nociceptive Pain
Nociceptive pain is caused by past or ongoing tissue injury in the form of mechanical, thermal or chemical insults to the patient. Tissue damage activates pain receptors (nociceptors) in the skin, soft tissue, skeletal muscle, bone and certain viscera as a warning signal to the body. Signals from nociceptors travel via peripheral sensory neurons and synapse within the dorsal horn of the spinal cord. Following pathways through the brainstem and thalamus, the pain signal is then interpreted by the somatosensory cortex. This process makes up the so-called ascending pathway of pain and is the therapeutic target of opioids. The interpretation and response to pain is heavily modulated by a number of factors including a subsequent descending pathway of inhibition of pain transmission from the brainstem to dorsal horn neurons. N-Methyl-d-aspartate (NMDA) receptors at the level of the dorsal horn of the spinal cord play an important role in this pathway. As signals continue to be received from the ascending pathway, NMDA receptors are activated and decrease central inhibition of the transmission of ascending pain signals from the periphery. This positive reinforcement of the pain signal is key to the continued sensation of pain. Importantly, μ-opioid receptor activity also increases NMDA activity, playing a central role in the development of opioid tolerance and opioid refractory pain. NMDA antagonists, therefore, have been shown to reduce opioid use through reduction in tolerance and by encouraging inhibition of pain signaling via the descending pathway (Collins and Walker 2006).
Important subtypes of nociceptive pain are somatic and visceral pain. Somatic pain occurs in response to inflammation and damage to the soft tissue, muscle, skin and bone. It tends to be well localized and described as a dull, aching pain or a sharp, stabbing sensation that is worse with movement. Visceral pain is caused by direct stimulation of afferent nerves due to tumor infiltration or inflammation of some, but not all, viscera. In contrast to somatic pain, visceral pain is poorly localized and is sometimes referred to another body site. Patients often describe visceral pain as dull, achy or squeezing (Friedrichsdorf and Kang 2007). Control of either type of nociceptive pain is dependent on treatment or amelioration of the underlying cause along with use of anti-inflammatory drugs and opioids.
9.3.2 Neuropathic Pain
Neuropathic pain results from disruption of neuron signaling pathways in the peripheral, autonomic, and central nervous system and is caused by direct nervous system injury or indirect injury due to medications such as vincristine (Baron et al. 2010). Nerve injury results in inflammatory mediator signaling which increases sodium channel expression on injured and surrounding neurons. The threshold of activation of the sodium channels is lowered which triggers nociceptor activation as well. Additionally, as in nociceptive pain, continued signaling from damaged afferent peripheral nerves leads to over activation of NMDA receptors with a resultant decrease in the inhibitory tone of the descending pathway. In addition to NMDA activity, serotonin and norepinephrine also play a role in the modulation of neuropathic pain, thus underlining the importance of NMDA antagonists (i.e., ketamine) and serotonin and norepinephrine reuptake inhibitors (i.e., SNRIs such as duloxetine, venlafaxine, desvenlafaxine) in the treatment of this pain (Portenoy et al. 2005). Neuropathic pain is described as numbness and tingling, itching, burning, “pins-and-needles” sensations, and sharp, shooting pain. Neuropathic pain can also present with sensory disturbances like allodynia and temperature sensitivity, causing dramatic pain with even minor, otherwise normal stimuli (Chong and Bajwa 2003).
9.4 Pharmacologic Treatment of Pain
A summary of general principles for pain management is provided in Table 9.1. Detailed discussion and case examples are given below. Practitioners should utilize pain assessment tools, expectation for length of pain control required, and the patient’s individual pain history to help guide decisions in pain management while continuing to reassess the patient for pain control and monitoring of side effects after initiation of pain medications.
Table 9.1
General analgesic principlesa
1. Assess pain. Pain is a subjective feeling (ask the patient or use a developmentally appropriate pain assessment scale – see Sect. 9.2) |
(a) Determine previous pain history and management, current medications, allergies |
(b) Explore contributing factors (e.g. disease course, anxiety, age, development, temperament) |
2. In opioid-naïve patients, start with short-acting opioids to control acute, moderate to severe pain. Do not use long-acting opioids to control acute pain |
3. When titrating or changing opiate dose, start by calculating the previous day’s total opioid requirement in oral morphine equivalents (OME) |
(a) Since all opioids produce analgesia by the same mechanism, they will produce similar degrees of analgesia if provided in equianalgesic doses (see Sect. 9.4.5) |
4. Determine if dose is adequate for pain control, and increase as needed (see Sect. 9.4.2) |
5. Choose opioid that will be used and dose adjust for incomplete cross-tolerance if necessary (see Sect. 9.4.5) |
(a) Typically, the only reasons to change from one opioid to another are side effects or renal failure |
(b) When rotating, decrease dose by 25–50 % to correct for incomplete cross-tolerance |
6. Determine route that opioid will be given. IM administration is rarely, if ever, indicated |
(a) Rectal = oral = sublingual dosing and SC = IM = IV dosing |
7. Determine a dosing schedule |
(a) Use only short-acting prn doses until a sense is gained as to how much opioid is needed |
(b) Once stable daily needs are determined (and need for pain medication is expected to persist), consider giving 66–75 % of daily OME as a long-acting opioid (see Sect. 9.4.3) |
(c) For patient-controlled analgesia (PCA) dosing see Sect. 9.4.6 |
8. Determine breakthrough dose for acute pain not controlled by the long-acting medication (see Sect. 9.4.4) |
(a) Use same opioid for short- and long-acting when possible |
(b) Give 10–15 % of total daily long-acting dose as breakthrough dose q3h prn |
9. Manage side effects as they arise (see Sect. 9.4.7) |
(a) Constipation is typically treated prophylactically |
9.4.1 World Health Organization Pain Ladder
The World Health Organization (WHO) created a three-step pain ladder as a basic approach to pain management in children with cancer (Fig. 9.2). A fourth step has also been developed for cancer pain not responding to pharmacotherapy alone and therefore requiring adjunct therapies such as interventional anesthesia. Morphine is the opioid used in the algorithm due to its ease of administration, tolerability, wide availability and well-understood side effects (Sirkia et al. 1997). Substitution with a different opioid is acceptable. The ladder is based on four major principles:
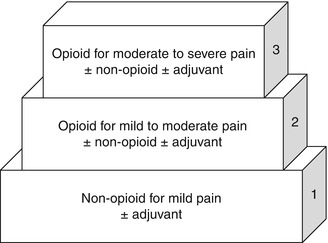

Fig. 9.2
World Health Organization three-step pain ladder
1.
By the ladder: a stepwise approach should be taken on the escalation of analgesics depending on the severity of symptoms.
2.
By the clock: scheduled analgesics should be given for steady state blood concentrations with appropriate as-needed (PRN) dosing.
3.
By the mouth: the least invasive route of administration should be used that is convenient and cost-effective with effective pain control.
4.
By the child: treatment plans should be individualized to the child’s pain level and response to therapy.
For mild pain (step 1), a nonopioid analgesic should be primarily utilized. Ibuprofen and acetaminophen have been established for analgesia in children >3 months of age; newborn trials have found these agents to be ineffective (Berde and Sethna 2002). Although not evidence-based, nonsteroidal anti-inflammatory drugs (NSAIDs) are generally avoided in pediatric oncology patients due to reversible antiplatelet and fever-masking effects.
Step 2 in the WHO pain ladder calls for weak opioid therapy with the consideration of adjuvants such as combined opioid-NSAIDs. Step 2 has not been well characterized and calls for the use of pain medications that are controversial in pediatrics (Tremlett et al. 2010). Codeine, for example, should be avoided in children due to concerns for both efficacy and safety. Genetic variability in cytochrome P450 isoenzyme 2D6 (CYP2D6) significantly alters the ability to metabolize the drug into its active metabolite, morphine. Roughly one-third of Caucasian children have limited ability or are unable to metabolize codeine to morphine, and codeine-related deaths have been reported in children with CYP2D6 alleles associated with ultrarapid metabolism (Williams et al. 2002; Kelly et al. 2012; Anderson 2013). The latter has led to a Food and Drug Administration (FDA) black box warning which advises that codeine is contraindicated in children after adenotonsillectomy for obstructive sleep apnea (Kuehn 2013). Additionally, medications such as tramadol have not been well studied in pediatrics, have a number of drug-drug interactions and reach a ceiling analgesic effect (Zernikow et al. 2009). Fixed combination products, such as acetaminophen/oxycodone and acetaminophen/hydrocodone, cause difficulty in dose escalation of the opioid due to dose limitations from the nonopioid component.
Step 3 in the WHO pain ladder calls for opioid therapy with consideration of an adjuvant. Several studies have demonstrated the efficacy of opioids in pain treatment. Although adjuvant therapy can be considered for the treatment of certain types of pain, a prospective non-randomized study in pediatric oncology patients demonstrated little additional analgesic benefit from the addition of nonopioids in children already receiving opioids (Zernikow et al. 2006). Further, analgesia was not improved when NSAIDs were added to a standing regimen of opioids, and the benefit of NSAIDs given for >7 days has not been established (McNicol et al. 2005). Lastly, children with cancer are at increased risk of NSAID-related bleeding as well as renal and gastrointestinal toxicity due to concurrent use of medications required for treatment of their tumors.
9.4.2 Intermittent Opioid Use
In opioid-naïve pediatric oncology patients, it is important to start with short-acting opioids to control acute, moderate to severe pain. Long-acting opioids should not be used for acute pain as they are designed to slowly release medication over a long period of time, generally 12 h. In children it is important to choose the type, preparation, dose and route of opioid such that the patient can comply with therapy. For example, intramuscular doses are avoided due to pain associated with administration and bleeding risks in patients with coagulopathy. Dosing depends on the route of administration, which in turn dictates the time to onset of medication effect. Rectal, sublingual and oral dosing are equivalent and have maximal analgesic onset within 30–45 min. Subcutaneous, intramuscular and intravenous (IV) dosing are also equivalent, but the onset of action is faster, typically within 15 min (Mercadante 2012). When switching between oral and IV routes, dosing must be adjusted using a standardized equianalgesia table (Table 9.2). It is important to remind patients changing from IV to oral dosing that while the analgesic effect is expected to be similar between routes, the time to onset of analgesia is not.
Table 9.2
Opioid equianalgesia conversions
Medication | Parenteral (mg) | Oral (mg) |
|---|---|---|
Morphine | 10 | 30 |
Oxycodone | – | 20 |
Hydromorphone | 1.5 | 7.5 |
Oxymorphone | 1 | 10 |
Fentanyl | 0.1a | – |
Short-acting doses are given as needed until a clear sense of the total daily opioid dose required to keep the patient comfortable is established. Once stable daily needs are determined and the need for pain medication is expected to persist, long-acting doses should be considered. Unless there are contraindications such as renal failure, morphine is generally used as the first-line opioid. Initial doses should be calculated using a dose-per-kilogram basis (Table 9.3). Doses should be increased by 25–30 % for uncontrolled pain.
Table 9.3
Pain management algorithma
Pain level | Rescue medication | Sustained medication | |
|---|---|---|---|
Step 1 | Mild pain | Start rescue medication: | Consider sustained therapy: |
Start a nonopioid analgesic (NSAID or acetaminophen) as needed | Consider a standing dose for short period of time | ||
Step 2 | Moderate pain | Start or escalate rescue medication: | Consider sustained therapy: |
Acetaminophen with hydrocodone | Acetaminophen with hydrocodone (same dosage on scheduled basis) | ||
Step 3 | Severe pain | Start or escalate rescue medication: | Start or escalate sustained medication: |
Morphine oral immediate-release preparation | Morphine oral immediate-release preparation | ||
(a) Opioid naïve: 0.2–0.3 mg/kg/dose q3-4 h prn | (a) Opioid naïve: 0.2–0.3 mg/kg/dose q3-4 h prn | ||
(b) Opioid exposed: increase previous dose 25–50 % for moderate pain and 50–100 % for severe pain | (b) Opioid exposed: total daily morphine dose divided every 3–4 h prn | ||
If meant for prolonged use, consider sustained-release morphine formulation | |||
Use of methadone or fentanyl patch should be in conjunction with palliative care or pain specialist | |||
Step 4 | Severe-persistent pain | Start or increase rescue medication: | Initiate or escalate sustained medication: (a) Transition sustained medication to IV equivalent (b) Consider an infusion |
Morphine: IV, SC (a) Opioid naïve <6 months: 0.05–0.1 mg/kg/dose q3-4 h prn (b) Opioid naïve >6 months: 0.1–0.2 mg/kg/dose every 3–4 h prn (c) Opioid exposed: previous total daily oral dose converted to IV morphine equivalent and divided by frequency | |||
Consider patient-controlled analgesia (PCA) with morphine: (a) Opioid naïve: basal infusion, 0–0.02 mg/kg/h; demand dose, 0.015–0.02 mg/kg/dose; lockout interval, 5–15 min (b) Opioid exposed: basal infusion, daily dose divided by 24 h; demand dose, 50–100 % of hourly dose; lockout interval, 5–15 min |
Case 1: Initiating Opioid Therapy
A 10-year-old, 30 kg boy presents to your outpatient clinic with a 3-month history of progressive right hip and knee pain. CT scan shows a pelvic lesion concerning for Ewing sarcoma, and now you and the family are planning an outpatient work-up of his tumor prior to starting therapy. The mother reports that he has been using five doses a day of acetaminophen for pain relief. Eric says he feels like he’s being “stabbed in the leg” and rates his pain as 8 of 10 with little relief from the acetaminophen. He has no drug allergies. They are asking if he can try something else for pain relief.
Plan
Looking at the WHO pain ladder, you decide to move up to opioids for moderate pain. Since he will be outpatient, you decide on an oral route, noting the patient prefers tablets to liquid medications. You decide to start with immediate-release morphine at a dose of 0.2–0.3 mg/kg every 3 h as needed (Table 9.3).
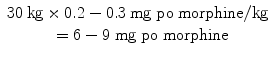 You note that the smallest tablet preparation of immediate-release morphine is 15 mg, so you instruct him to take 7.5 mg (0.5 tab) every 3 h as needed and to call if it is ineffective. Of note, preparations including hydrocodone rather than oral morphine would also be reasonable in this case.
You note that the smallest tablet preparation of immediate-release morphine is 15 mg, so you instruct him to take 7.5 mg (0.5 tab) every 3 h as needed and to call if it is ineffective. Of note, preparations including hydrocodone rather than oral morphine would also be reasonable in this case.
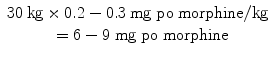
9.4.3 Long-Acting Opioids
One should consider the use of long-acting opioids when a stable daily dose of opioids is achieved and a persistent use of pain medication is expected. As described above, long-acting agents should not be used for acute pain as they slowly release medication. Long-acting opioids allow for around-the-clock analgesia and are used in combination with short acting opioids that serve to manage acute or breakthrough episodes of pain. The benefits of long-acting opioids include less use of breakthrough medication, improved sleep at night, improved comfort upon waking from sleep and less drowsiness.
When determining the amount of long-acting opioids, it is important to calculate the previous day’s total opioid requirement (oral morphine equivalent or OME). Long-acting opioids should be dosed at 1/2 to 2/3 of the total OME. Slow-release morphine is used as first-line treatment and generally prescribed every 8–12 h. Doses should be reevaluated and titrated upwards if optimal pain control is not reached. This often occurs with disease progression or, less often, as a result of opioid tolerance.
Fentanyl patches and methadone are also effective as long-acting agents but are generally reserved when therapy with morphine or oxycodone has been unsuccessful or side effects have precluded their use. Equianalgesic conversions of fentanyl and methadone can be complicated and vary based on the total amount of OMEs. Methadone has unique pharmacokinetic properties that must be taken into consideration due to a long half-life requiring initially frequent dosing but later only every 12–24 h dosing. Methadone is potentially beneficial as it has less pruritus side effect and also may help treat concomitant nausea. A fentanyl patch can be a convenient way to provide pain medication delivery in the patient unable to easily take oral medications. Fentanyl and methadone should be prescribed by clinicians experienced in their use.
Case 1 (Continued) Starting Long-Acting Opioids
You see Eric 2 days later and he tells you the morphine is working well. Eric says his pain drops from 8 of 10 to 3 of 10 within about 30 min of taking the medication, and the pain relief lasts for 3–4 h. His mother says he uses seven doses a day, but that he has excellent pain control. However, Eric does say that when he wakes up from sleeping his pain is really bad and takes some time to come back under control. Thus, his mother has been waking him up at night to give him the morphine. His mom is tired, but she tells you she’s willing to do whatever it takes to keep him comfortable.
Plan
You decide that Eric would best be served with a long-acting opioid. To start, you calculate his total daily opioid requirement.
 You then determine that about 2/3 of his daily dose should be in his long-acting medication. Taking 2/3 of 52.5 mg, you decide that approximately 35 mg of morphine per day should be given in a long-acting form. Since morphine has worked so well for him, you choose sustained-release morphine as your long-acting agent. Noting that it comes in 15 mg tablets, you prescribe 15 mg by mouth twice a day, which will provide 30 mg per day in a long-acting form. You take care to advise Eric and his mother that this should be taken roughly every 12 h regardless of his pain level and that this medication will not help with breakthrough pain. He will also need a breakthrough dose; instructions for this calculation are in Sect. 9.4.4.
You then determine that about 2/3 of his daily dose should be in his long-acting medication. Taking 2/3 of 52.5 mg, you decide that approximately 35 mg of morphine per day should be given in a long-acting form. Since morphine has worked so well for him, you choose sustained-release morphine as your long-acting agent. Noting that it comes in 15 mg tablets, you prescribe 15 mg by mouth twice a day, which will provide 30 mg per day in a long-acting form. You take care to advise Eric and his mother that this should be taken roughly every 12 h regardless of his pain level and that this medication will not help with breakthrough pain. He will also need a breakthrough dose; instructions for this calculation are in Sect. 9.4.4.

9.4.4 Breakthrough Dosing
Breakthrough dosing of pain medications should be used for acute pain that is not controlled by a long-acting medication. When possible, the same opioid should be used as the short- and long-acting agent. This dose should be calculated as 10–20 % of the total daily long-acting dose every 3–4 h as needed. All patients should have access to a breakthrough medication. An adult study demonstrated that up to 67 % of cancer patients with well-controlled pain have transitory episodes of breakthrough pain requiring treatment (Bruera and Neumann 1999). Younger children with cancer tend to experience more breakthrough pain than older children (Friedrichsdorf et al. 2007).
If more than three breakthrough doses are needed in 24 h, consideration should be given to increasing the long-acting opioid dose. This should be done by calculating the daily OME from the long-acting opioid and from the total amount of breakthrough medicine required. A new long-acting dose can then be calculated using the principles listed in Sect. 9.4.3. A new, higher, breakthrough dose should be calculated as 10–20 % of the new, total daily long-acting dose.
Case 2 Calculating Breakthrough Dosing
You are taking care of a 50 kg girl with osteosarcoma of the proximal tibia. She is now 6 days post gross total resection of her tumor with a limb-sparing procedure. She has been on morphine by patient-controlled analgesia (PCA), and from a surgical standpoint, she is ready for discharge. Her morphine PCA settings have been a continuous rate of 1 mg/h with a demand dose of 1.5 mg every 15 min. She has been receiving about eight demand doses per day for the past 2 days and notes good pain control. The surgical team is asking you to determine an outpatient pain plan. She requests that you attempt to keep things in pill form.
Plan
The first step is to determine the total daily morphine requirement. Each day she receives 24 mg (1 mg/h) from her continuous rate and 12 mg (1.5 mg × 8 doses) from her demand, giving a total dose of 36 mg of IV morphine/day. Next you must decide on the oral formulation. Since morphine has worked well for her, you decide that you will use sustained-release morphine as your long-acting agent and immediate-release morphine for breakthrough.
To calculate the proper dose, you then need to convert the medication into OMEs (Table 9.2, see Sect. 9.4.5):
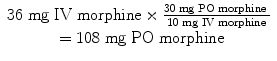 The next step is to determine the amount of medication to place in long-acting form. In general, you would choose to take 2/3 of the total daily dose. Thus, 2/3 of 108 mg po morphine is about 72 mg po morphine. Noting that sustained-release morphine comes in 30 mg tablets given every 12 h, you decide to use 30 mg twice daily as the long-acting dose.
The next step is to determine the amount of medication to place in long-acting form. In general, you would choose to take 2/3 of the total daily dose. Thus, 2/3 of 108 mg po morphine is about 72 mg po morphine. Noting that sustained-release morphine comes in 30 mg tablets given every 12 h, you decide to use 30 mg twice daily as the long-acting dose.
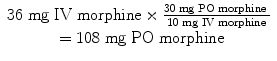
Next you need to calculate the breakthrough dose. Knowing that you are now going to give 60 mg per day as a long-acting dose, you can estimate the breakthrough dose at 10–20 % of the long-acting dose. This gives you a dose somewhere between 6 and 12 mg of immediate-release morphine. Recognizing that immediate-release morphine comes in 15 mg tablets, you choose a breakthrough dose of 7.5 mg.
Hence, you have settled on the following plan:
1.
Sustained-release morphine 30 mg po twice a day
2.
Immediate-release morphine 7.5 mg po every 3 h as needed for breakthrough pain
9.4.5 Opioid Rotation and Equianalgesia
When titrating or changing opiates, it is important to start by calculating the previous day’s OME. All opioids produce analgesia by the same mechanism; thus, if provided in equianalgesic doses, they will produce the same degree of analgesia (Table 9.2). The most common reasons to change from one opioid to another are side effects and poor response to pain control despite appropriate titration (Drake et al. 2004). Other indications for opioid rotation include renal failure, problematic drug-drug interactions, preference or need for different route of administration or financial considerations.
When rotating opioids it is important to consider incomplete cross-tolerance. This concept takes into account that tolerance developed to one opioid does not imply complete tolerance to another. Also, the new drug may be more effective due to differences in drug bioavailability or potency (Pasero and McCaffery 2011). To account for this phenomenon, a dose reduction by 25–50 % should occur when rotating opioids (Indelicato and Portenoy 2002). In most cases a 25 % reduction is appropriate but practitioners should use clinical judgment in determining how much of a dose reduction should occur as a result of incomplete cross-tolerance. For example, in the case of a patient who has undergone rapid increases in opioid dosing for pain subsequently deemed to be allodynia, a dose reduction of 50 % may be more appropriate.
Case 3 Opioid Switching
You are taking care of a 50 kg young man with newly diagnosed leukemia. He’s been using morphine 5 mg IV every 3 h as needed for bone pain. Although he notes good pain relief, he has been itching uncontrollably. He asks you if there is anything you can do to help with his pain but keep him from itching.
Plan
This is one of the most common reasons to switch opioids. Although you could try a medication to treat the itching, changing the offending agent avoids polypharmacy. You decide that hydromorphone would be a nice alternative. To calculate a dose, you first need to determine the equianalgesic dose of hydromorphone and morphine (Table 9.2):
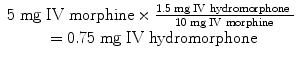 The last step is to account for incomplete cross-tolerance. A dose reduction of 25 % would provide a dose of about 0.56 mg of IV hydromorphone. Thus, an order for 0.6 mg of IV hydromorphone every 3 h as needed for pain would be appropriate.
The last step is to account for incomplete cross-tolerance. A dose reduction of 25 % would provide a dose of about 0.56 mg of IV hydromorphone. Thus, an order for 0.6 mg of IV hydromorphone every 3 h as needed for pain would be appropriate.
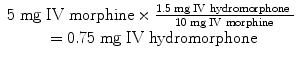
9.4.6 Patient-Controlled Analgesia Calculations
Patient-controlled analgesia (PCA) is indicated for use when pain is not controlled with oral medications, there is inability to take oral medications, there is possibly poor gastrointestinal absorption of pain medications, or if the child or parent has a preference for the PCA. Studies have demonstrated PCA effectiveness in postoperative pain, but PCA use is additionally effective in burns, mucositis, bone marrow transplant and sickle cell disease (Gaukroger et al. 1991; Mackie et al. 1991; Collins et al. 1996a; Dunbar et al. 1995; Trentadue et al. 1998; Walder et al. 2001). Children as young as 6 years are able to independently provide effective postoperative pain control when taught appropriate PCA use (Berde et al. 1991).
General principles and dosing of PCA are listed in Table 9.3. Opioid naïve patients should be started at low doses of medication. Lockout intervals, which represent how often a patient can push their PCA button to receive medication, should range between 5 and 15 min. Children currently receiving opioids should have their total daily OME calculated and a proportion of that dose (usually 2/3) divided over 24 h to give a continuous PCA rate. Demand (or breakthrough) doses for these patients should range from 50 to 100 % of their total hourly doses. PCA dosing plans should be frequently reassessed and titrated as necessary.
Initiation of PCA requires close monitoring of respiratory rate, oxygen saturation, blood pressure, heart rate, pain score, sedation score and nausea. Continuous pulse oximetry should be considered when PCA is started and naloxone should be available. If the child becomes somnolent, difficult to arouse, has a respiratory rate <8 or oxygen saturation <92 % with respiratory rate <12, or pinpoint pupils, administration of the opioid should be stopped and supplemental oxygen given along with naloxone. Doses can be cautiously restarted at 50 % of the original dose once the patient is alert and if they are experiencing pain. Many institutions have developed protocols for authorized agent-controlled analgesia (AACA) for children <6 years which can include either nurse-controlled or caregiver-controlled analgesia as long as the caregiver is consistently available, competent, and properly educated and the authorized agent is designated in the medical order (Wuhrman et al. 2007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree