Breast cancer is the most common cancer in women and it is estimated that one in eight women in the United States will develop breast cancer in their lifetime. The American Cancer Society reported that in 2013 an estimated 232,340 new cases of invasive disease and 39,620 breast cancer deaths would occur.1 Every year more than 40,000 women die from breast cancer, making it the second leading cause of cancer-related deaths in women. Only lung cancer accounts for more cancer deaths in women. Historically, the increase in breast cancer incidence was thought to be a reflection of changes in reproductive patterns, such as delayed childbearing, no breast feeding, and giving birth to fewer children, which are recognized as risk factors. Since the early 1980s, the incidence of breast cancer among postmenopausal women was steadily rising due to improved detection with the widespread use of mammography. The rates stabilized in the late 1990s and then started to decrease; it has been hypothesized that the decline occurred due to a reduced use of hormone replacement therapy following the 2002 publication of the Women’s Health Initiative randomized trial results.2,3 In contrast the incidence of breast cancer in younger women has remained stable. Although breast cancer is a rare disease in young women, it is the most common cancer occurring in the age group. When it occurs in women <35 years, it brings forth unique clinical challenges for the clinician and personal challenges for the patient, especially when associated with pregnancy.4,5 With a rapidly aging population in the United States, the number of breast cancer is expected to increase over the next 20 years. Fortunately, breast cancer mortality rates have declined continuously since 1990, despite the larger and older U.S. population.6 Part of this decline in mortality is attributable to early diagnosis; however, a large part is due to advances in cancer treatment and the incorporation of adjuvant systemic therapy.
Breast cancer therapy requires a multidisciplinary team consisting of surgeons, medical oncologists, and radiation oncologists. All three specialties have seen significant advances, and each component of these treatments has been shown to independently offer survival benefits for selected patients. For patients with early-stage breast cancer, the outcomes associated with breast-conservation surgery, sentinel lymph node dissection, radiation, and systemic treatments are excellent. Treatment advances have also improved the prognosis for patients with more advanced disease at presentation. A particularly exciting such advance has been the use of trastuzumab and other anti-HER2-directed therapies, including lapatinib, pertuzumab, and ado-trastuzumab, for patients with HER2-positive disease. In the future, additional molecularly targeted treatments are likely to further improve breast cancer outcomes.
This chapter of this textbook consists of an overview highlighting the role of systemic therapy in the following settings: early-stage disease, locally advanced breast cancer (LABC), inflammatory breast cancer (IBC), special populations (elderly, male, pregnant), ductal carcinoma in situ, and in the preventative setting. In this chapter, important aspects of biology, pathogenesis, risk factors for the disease, and prognostic and predictive factors, including the molecular classification of disease, are discussed as they relate to the role of chemotherapy, hormonal therapy, and targeted therapy in breast cancer.
For patients with stage I to III breast cancer, the goals of treatment are curative. Treatment decisions are ultimately made with regard to patient risk of relapse, and comorbidities, after weighing the potential benefits and harms of therapy, and based upon patient’s personal preferences. Often, several extended discussions between the patient and a multidisciplinary team including medical oncology, surgical oncology, plastic surgery, and radiation oncology are needed to determine the best treatment plan. Multimodality therapy (Fig. 82-1) has had a profound impact on the clinical outcomes of breast cancer as it has allowed for improved disease-free survival and overall survival. Surgery and radiation have important roles in the curative management of most breast cancers and greatly determine the level of local control achievable. Despite advances in locoregional therapy, a significant number of women with early and locally advanced breast cancer go on to develop metastatic disease within 5 to 10 years after their diagnosis. When metastatic breast cancer develops, the condition is incurable and requires lifelong systemic therapy to control the disease, palliate symptoms, and prolong survival. The current thought is that metastasis has likely occurred prior to initial clinical evaluation and diagnosis of breast cancer in the form of micro-metastases that are undetectable by available technologies. Therefore, systemic therapies are useful to eradicate the potential microscopic metastatic disease present and prevent recurrence. The complex aspect of administration of systemic therapy is the inability to detect occult metastatic disease accurately and the difficulty in selecting patients likely to benefit most from systemic treatment.
FIGURE 82-1:
Overview of multimodality therapy for breast cancer. Abbreviations: ER, estrogen receptor; PR, progesterone receptor; BCS, breast conservation surgery; NAC, neoadjuvant chemotherapy; SLND, sentinel lymph node dissection; ALND, axillary lymph node dissection. aAnthracycline and taxane–based therapy is preferred. For Her2-positive tumor, add dual anti-HER2 blockade to the taxane portion. bThe extent of surgery is determined by response but always includes an axillary lymph node dissection (ALND). In inflammatory breast cancer, the surgery is always mastectomy + ALND. cComplete planned chemotherapy regimen if not completed preoperatively. Add endocrine treatment if ER-positive and/or PR-positive. Complete up to 1 year of trastuzumab in HER2-positive disease; may be administered concurrently with radiation therapy and with endocrine therapy if indicated. dAdjuvant chemotherapy is indicated in triple negative and HER2-positive tumors >5 mm. In hormone receptor positive, HER2 negative tumors, chemotherapy is beneficial in patients with a high recurrence score on Oncotype-DX testing and is currently offered as standard of care in patients with intermediate recurrence score. eWhole breast radiation +/− chest wall and lymph node basins as clinically indicated. For inflammatory breast cancer, radiation should include chest wall, level I/II axillary lymph nodes, supraclavicular nodes, and internal mammary lymph nodes if involved. (Adapted with permission from NCCN guidelines and the ASCO-SEP Medical Oncology Self-Evaluation Program.)
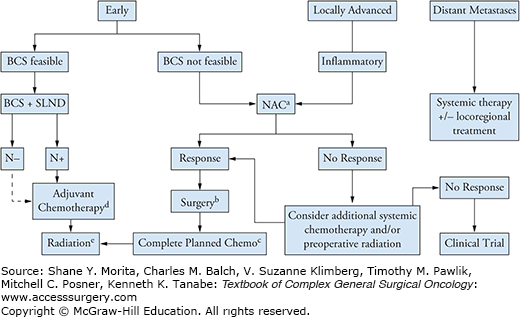
For invasive breast cancer, in stages I to III, the treatment intent is curative. Systemic treatments are added to the regimen of locally advanced or early-stage breast cancer based upon a balance between the risk of distant metastasis and the benefit of therapies to reduce the risk of relapse early in the disease course (within the first 5 to 10 years). Data from a meta-analysis of adjuvant chemotherapy trials conducted by the Early Breast Cancer Trialists Collaborative Group indicate that systemic therapy provides a survival benefit for patients with lymph node–positive disease, and also those with lymph node–negative disease, including those with estrogen receptor (ER)-positive tumors.7 In addition, patient preferences are given strong consideration. Patients may hold personal beliefs that the reduction in risk of recurrence with adjuvant therapy does not outweigh the adverse effects of treatment, particularly chemotherapy. Many times long discussions aimed at empowering patients with the most accurate information regarding the risks and benefits of each treatment are needed to foster educated and individualized decision making. With higher stage of disease, the likelihood of developing systemic recurrence increases. In addition, to the extent of disease present at diagnosis, the underlying biological characteristics of the tumor are key to determine the risk of recurrence or existence of micrometastatic disease. Biomarkers, such as ER, PR, and HER2, affect prognosis and predict response to therapy as discussed in the next section. Furthermore, age is an independent prognostic feature and younger women are at a higher risk of local and distant recurrence. In the setting of a young patient population, adjuvant systemic therapy is offered at a lower risk/benefit threshold. In comparison, for women older than age 70, there is no strict guideline as few of them are included in clinical trials and decisions to proceed with systemic treatment should consider patient comorbidities and toxicity related to treatments.
Breast cancer is a biologically heterogeneous disease. For many years, patient age, axillary lymph node status, tumor size, and histological features (tumor grade, presence of lymphovascular invasion), hormone receptor status, and HER2 status were the major factors used to assess prognosis and select therapies. Recent advances in genomics and molecular biology techniques have permitted more comprehensive assessments of the underlying genetic heterogeneity. DNA microarray expression profiles have led to classification of breast cancer into molecular subtypes that correspond with distinct prognostic groups based upon biology and aggressiveness of the disease.8,9 Interestingly, these molecular classes are strongly influenced by the genes controlling expression of the ER and the ERBB2 receptor HER2/neu.10
The molecular profiles of estrogen-enriched cancers tend to be similar to those of luminal ductal cells, whereas estrogen-negative breast cancers have molecular phenotypes similar to cells in the basal layers of the breast ductal epithelium. Tumors that overexpress HER2/neu have unique signatures. Recent molecular classifications often group diseases into four classes (Table 82-1) that overlap but are not synonymous with variations in ER, progesterone receptor (PR), and HER2/neu status: luminal A tumors, which include most ER-positive, PR-positive, HER2-negative tumors; luminal B tumors, which include most ER-positive, PR-positive, and some HER2-positive tumors; HER2 enriched, which include most of HER2-positive tumors; and, finally, basal cancers, which overlap significantly with triple-negative (ER-negative, PR-negative, HER2-negative) breast cancers.
Breast Cancer Molecular Subtypes: Classification and Prognosisa
| Subtype | Gene Expression Pattern | Clinical Features | Treatment Response and Outcome |
|---|---|---|---|
| Luminal | High expression of hormone receptors and associated genes (luminal A > luminal B) |
|
|
| HER2 | High expression of HER2; low expression of ER and associated genes |
| Overall poor prognosis, but responds to trastuzumab and anthracycline-based chemotherapy |
| Basal | High expression of basal epithelial genes, basal cytokeratins; low expression of HER2, ER, and associated genes |
|
|
Luminal A disease expresses more ER-related genes, lacks HER2 overexpression, and has low proliferation rates; it carries the best prognosis and predicts response to hormonal therapy. Luminal B tumors express more proliferation and HER2-related genes and often have a higher grade and a worse prognosis compared to luminal A. Many of these tumors are sensitive to chemotherapy. Basal-type cancers tend to be more chemoresponsive but are nevertheless an aggressive molecular subtype with an overall poor prognosis. Finally, the inherent aggressive biology of HER2-positive disease has been transformed with the use of trastuzumab and other HER2-directed therapies. It is well known that the various subtypes of breast cancers have different prognoses.9,11–13 More recently, it has been shown that the different molecular subtypes have not only different long-term survivals but also different sensitivities to neoadjuvant chemotherapy.14 Among triple-negative breast cancers which are well known to be aggressive and lack targeted therapies, even more subtypes have been identified including two basal-like (BL1 and BL2), an immunomodulatory (IM), a mesenchymal (M), and a mesenchymal stemlike (MSL).15
In addition to classification, molecular markers are utilized to select patients for systemic treatment (i.e., chemotherapy, endocrine therapy, and targeted therapy) and to predict those who are most likely to benefit from the therapies. The quintessential example of a predictive marker is ER; since its discovery all breast tumors are pathologically assessed for ER and patients with ER-positive tumors benefit from antihormonal treatments such as tamoxifen. A second important predictive factor was discovered in 1985 as the human epidermal receptor 2 (HER2) or erbB2 protein and is found to be amplified in 20% of breast cancers leading to protein overexpression.16,17 Trastuzumab is a humanized monoclonal antibody against the extracellular domain of the surface receptor and is a very effective treatment in HER2-positive breast cancer in both the metastatic and adjuvant settings (adjuvant therapy as discussed later).18 HER2 testing by immunohistochemistry (IHC) to measure protein expression and/or fluorescent in situ hybridization (FISH) to measure HER2 gene copies is standard for all breast tumors and is predictive of response to targeted therapy.19
To help physicians and patients make treatment decisions and understand prognosis, Adjuvant! (www.adjuvantonline.com) was designed to estimate the benefit of adjuvant systemic therapy based upon patient age, comorbid conditions, tumor histological grade, hormonal receptor (HR) status, tumor size, and the number of involved lymph nodes.20 Systemic therapy is considered for all patients when the tumor size is larger than 0.5 cm regardless of nodal status, or when there is lymph node involvement regardless of tumor size. Factors that suggest greater benefit to chemotherapy include high histological grade, moderate to large size, lymph node involvement, triple negative disease, HER2-positive disease, high proliferation rate, and a high breast cancer recurrence score (RS) determined by Oncotype-DX testing. The only patients who may not be at sufficient risk to warrant systemic treatments are those with very small stage I tumors that do not express HER2/neu (Fig. 82-2).
FIGURE 82-2:
Guidelines for adjuvant systemic therapy for (A) hormone receptor–positive, HER2-negative disease, (B) hormone receptor–positive, HER2-positive disease, (C) hormone receptor–negative, HER2-negative disease, and (D) hormone receptor–negative, HER2-positive disease. Abbreviations: ER, estrogen receptor; PR, progesterone receptor; N+, node positive; HT, hormonal therapy; CT, chemotherapy; T, trastuzumab; RS, recurrence score. *No adjuvant therapy; consider hormonal therapy in hormone receptor positive tumors for disease prevention. (Adapted with permission from NCCN guidelines and the ASCO-SEP Medical Oncology Self-Evaluation Program.)
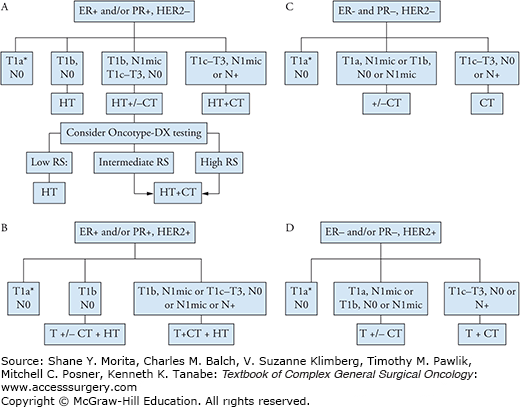
More recently, genomic products may be combined to provide a picture of the molecular phenotype of the individual’s cancer and thereby predict recurrence and/or response to chemotherapy and other systemic therapies. The Oncotype-DX assay (Genomic Health) is a 21-gene recurrence score assay that was originally designed to predict the recurrence of ER-positive, node-negative breast cancer after treatment with adjuvant endocrine therapy. The test determines 10-year risk of recurrence using formalin-fixed, paraffin-embedded tumor tissue. The expression of 21 genes is analyzed and a risk score (RS) is assigned based on a weighted algorithm. The assay was validated in 668 patient samples from ER-positive, node-negative, tamoxifen-treated breast cancer patients who were enrolled in the NSABP B-14 clinical trial.21 It has now been clinically adopted to determine whether women with high-risk, node-negative, HER2-negative, ER-positive breast cancer should receive adjuvant chemotherapy in addition to endocrine therapy, as it is able to predict the magnitude of benefit.22
The assay categorizes patients into three risk groups: (1) a low-risk group (RS, 0-18), distant recurrence risk of less than 10%; (2) intermediate-risk group (18 < RS > 31), distant recurrence risk between 10% and 20%; and (3) high-risk (RS > 31), distant recurrence risk greater than 20%. Chemotherapy is generally recommended for patients with intermediate and high RS.23 Other multigene assays have been identified such as Mammaprint and PAM50.
The National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of Oncotype-DX testing in hormone receptor–positive, node-negative patients to help identify patients who are predicted to obtain the most benefit from adjuvant chemotherapy and those who may require adjuvant hormonal therapy only.23 Tumors larger than 1.0 cm or tumors measuring 6 mm to 1.0 cm with unfavorable features including angiolymphatic invasion, high nuclear grade, or high histological grade should be tested in node-negative, hormone receptor–positive, and HER2-negative patients. Tumors with a high RS (>31) have a predicted benefit from chemotherapy in addition to hormonal therapy. Patients in the low-risk group (RS, 0–18) with a distant recurrence rate of less than 10% after 5 years of adjuvant tamoxifen are predicted to have marginal benefit if any from chemotherapy and should be offered hormonal therapy alone. Patients who are in the intermediate and high RS group (>18) and those who are node positive are currently been advised to receive adjuvant chemotherapy; the clinical utility of this test in these groups of patients with early-stage ER+ HER2/neu normal breast cancer is being further defined in clinical trials (TailorX; RxPONDER). In the prospective, randomized TAILORx clinical trial, patients assigned an RS (>10 but <26) were randomized to receive either chemotherapy and hormonal therapy or hormonal therapy alone. This trial enrolled more than 11,000 patients and closed in October 2010. Results are eagerly anticipated and will refine the utility of the test for the risk group. A trial for node-positive patients (RxPONDER; SWOG S1007) is currently enrolling patients to determine whether there is a subset of node-positive (1-3), HER2-negative, hormone receptor–positive, RS of 0–25, patients who may be spared from chemotherapy. As discussed later in this chapter, according to current guidelines, node-positive should be offered chemotherapy outside of a clinical trial.23
In the early 1900s, a German chemist named Paul Ehrlich first coined the term “chemotherapy” as the use of chemicals to treat disease.24 Surgery and radiotherapy had dominated the field of cancer therapy into the mid-1900s and eventually it was recognized that cure rates after radical local treatments were plateauing due to the presence of micro-metastases that until then had been unappreciated. In the 1960s, the concept of curing cancer with drugs was first introduced and the idea to combine drugs with locoregional therapy to address the issue of micrometastatic disease came to fruition and the field of adjuvant chemotherapy was formed. Many breast cancer patients who present with locoregional disease, especially if lymph node positive, will develop recurrences if only local therapies are used. Yet, a significant number will remain free of disease with regional treatment alone. These observations led to the clinical dilemma of determining which patients are unnecessarily exposed to the toxic effects of systemic treatments and which patients will gain the most benefit and cure. Still today, the main cause of death for patients with breast cancer is from complications due to and/or progression of metastatic disease. Adjuvant systemic therapies (chemotherapy or hormonal) benefit patients by treating potential micrometastasis and thereby curing breast cancer or at least delaying development of clinical metastasis. Chemotherapy drugs used to treat breast cancer gained their foundation first in the treatment of advanced, incurable disease and were later applied to the treatment of early breast cancer. This stems from Howard Skipper’s “cell kill” hypothesis and the inverse mathematical relationship between cell number and curability which suggests that the same drugs that are effective against advanced disease may work better in the adjuvant setting where there is less disease in the form of micrometastases.24,25 Therefore, when therapeutic agents are identified to improve outcomes for patients with metastatic breast cancer, they are often adapted into clinical studies and tested in the early breast cancer setting.
Treatment for breast cancer with cytotoxic agents will impair fertility. Therefore, premenopausal patients should be informed of the potential impact of chemotherapy on fertility. It is appropriate to consider fertility preservation prior to breast cancer treatment in young women who desire to bear children in the future. In general, a combination of two or more drugs is more effective than single agents. Combinations are devised keeping in mind the different mechanisms of action of the drugs that target tumor cells as well as the toxicities of each agent and they are combined to maximize the non-cross-resistant effects and minimize the overlapping toxicities. Therapies may be administered in the adjuvant (after surgery) and/or neoadjuvant (before surgery) settings. Depending on which regimen is selected, the duration of therapy may be from 3 to 6 months. Longer durations (>6 months) with the same drugs in combination have not proven efficacious. Dose reductions or delays are not recommended unless significant toxicity results. For manageable toxicities such as myelosuppression and nausea/vomiting, significant efforts should be made to manage toxicities without dose reductions via transfusions, growth factor support, and antiemetics.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has completed the largest and most extensive analysis of the benefits of chemotherapy. The group meets every 5 years and the publication from 2005 summarizes data from all adjuvant breast cancer chemotherapy trials since 1995 involving 14,000 women participants. There were 60 trials evaluating adjuvant chemotherapy versus no chemotherapy and 17 trials evaluating more specifically the combination of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) versus anthracycline-based chemotherapy. For younger women (under age 50) polychemotherapy has been found to reduce the risk of death by 30% and the risk of relapse by 37% compared to not using chemotherapy. For women older than 50 years, similar benefits but of less magnitude were seen with a 12% reduction in the risk of death and 19% reduction in the risk of relapse.
The most widely adopted regimens include combinations of an anthracycline (either doxorubicin or epirubicin) and a taxane (either paclitaxel or docetaxel). Anthracyclines inhibit topoisomerase II, preventing repair of DNA cleavage and leading to DNA breaks. Additionally, it is an antimetabolite that inhibits DNA and RNA synthesis and creates free oxygen radicals that damage DNA, proteins, and cell membranes. Anthracyclines have long been touted as the single most important and active drug in breast cancer since the 1970s.26 The EBCTCG analyses showed that anthracyclines modestly add further benefit when compared to non-anthracycline-based therapies such as CMF; the breast cancer mortality and overall mortality were found to be 3% at 5 years and 4% at 10 years in favor of anthracyclines with a 16% reduction in risk of death and a 11% reduction in the risk of recurrence.27 The widespread adoption of anthracycline-based adjuvant chemotherapy regimens during the 1980s and 1990s lead to an almost complete replacement of the CMF chemotherapy combination.27–29 The CMF combination is mostly used now in the adjuvant setting in special circumstances of low-risk disease, in patients with cardiac problems that prohibit them from receiving anthracyclines, and in cases of recurrent breast cancer with previous anthracycline exposure. The main toxicity of anthracyclines includes a risk of cardiotoxicity (1.5% to 3%), which can be acute or long-term. Acute cardiotoxicity is rare, not dose-related, symptomatic/asymptomatic, and spontaneously resolves. Two forms of long-term cardiotoxicity are recognized. Type I cardiac dysfunction is dose-related, often irreversible, and characterized by myocyte damage that is mediated by free radicals. In contrast, type II cardiac dysfunction is not dose-related, is highly reversible, and is not associated with structural damage.30 Of the two most commonly used anthracyclines—doxorubicin and epirubicin—the latter is less cardiotoxic but it must be administered at 25% to 50% higher doses in order to achieve the same clinical efficacy; therefore, the cardiac effects are likely similar. For this reason, neither anthracycline is thought to be safer than the other and the generally accepted dosing allows for up to 450 to 550 mg/m2 of doxorubicin, or up to 900 mg/m2 of epirubicin. An additional consideration is the risk of hematologic toxicity and development of leukemia (<1%).31 These risks are important to keep in mind since some early-stage breast cancer patients may be cured without the use of anthracyclines.
Improving on the outcomes observed with adjuvant anthracycline-based chemotherapy, two ideas came forward in the 1990s: (1) adding taxanes to the anthracycline backbone and (2) increasing the dose intensity of the anthracycline. Taxanes are microtubule inhibitors and have significant activity in chemotherapy-naive tumors as well as anthracycline-resistant tumors. A number of clinical trials have evaluated the use of taxanes in the treatment of early-stage breast cancer. An intergroup trial randomly assigned patients who had been treated with four cycles of doxorubicin and cyclophosphamide (AC) to receive either four cycles of paclitaxel or no additional chemotherapy. Results of that trial showed a disease-free survival benefit for patients treated with paclitaxel.32 These results are supported by those of a similar study conducted by the NSABP (the B-28 trial), which also found that patients who received four cycles of AC plus four cycles of paclitaxel had higher disease-free survival rates than those who received four cycles of AC alone.33 A meta-analysis of taxanes in 13 different studies described an improvement in disease-free survival (DFS) (hazard ratio (HR), 0.83; 95% confidence interval (CI), 0.79 to 0.87; p < .0001) and overall survival (OS) (HR 0.85; 95% CI 0.79 to 0.91; p < .0001).34 Either paclitaxel or docetaxel may be used at an optimal dose and schedule with equivalent outcomes. The timing and delivery of paclitaxel is important. The activity of docetaxel is less dependent on timing of therapy and this drug is administered on an every 3-week basis. In the ECOG 1199 trial, patients with lymph node–positive breast cancer were randomized to receive paclitaxel or docetaxel weekly or every 3 weeks after completion of an anthracycline-based regimen.35 Weekly paclitaxel and every 3-week docetaxel were associated with the most favorable outcomes in terms of disease control and had the least adverse effects. Every 3-week administration of paclitaxel was found to be an inferior dosing schedule.
Subsequent chemotherapy trials have investigated various combinations of taxane and anthracycline chemotherapy. An intergroup trial revealed that giving four cycles of AC followed by four cycles of paclitaxel in a dose-dense fashion improved disease-free survival rates relative to the usual 3-week dosing schedule of the same chemotherapy.36 Finally, the Breast Cancer International Research Group conducted a trial that randomly assigned patients with lymph node–positive breast cancer to receive either six cycles of docetaxel, doxorubicin, and cyclophosphamide (TAC) or six cycles of fluorouracil, doxorubicin, and cyclophosphamide (FAC) and found an improvement in disease-free survival rates among those given TAC.37
In the United States, several regimens (Table 82-2) are considered appropriate standard treatment for patients with high-risk disease features (e.g., lymph node–positive disease): dose-dense scheduling of AC followed by paclitaxel, weekly paclitaxel with FAC, 3-week FEC follow by docetaxel, or TAC. For patients with lower risk breast cancer who desire a shorter course of chemotherapy, giving four cycles of docetaxel and cyclophosphamide (TAC) has become more common after a recent randomized prospective trial found that this regimen produced better disease-free survival rates than did four cycles of AC.38 Each regimen has variable toxicities and selection is based on a balance between the potential risk and benefit of each. Clinicians may select a regimen for each individual patient with a goal to maximize benefit and minimize harm.
Adjuvant or Neoadjuvant Chemotherapy Options
| HER2-negative Diseasea | HER2-positive Diseaseb |
|---|---|
| Adjuvant or Neoadjuvant | Adjuvant |
|
|
| Neoadjuvantf | |
|
Triple-negative breast cancer (TNBC) lacks expression of estrogen and progesterone receptors (ERs and PRs) and overexpression or amplification of human epidermal receptor 2 (HER2) and is worth of some focused attention. It is associated with a younger age of onset, BRCA1 mutations, larger tumor size, higher grade tumors, high proliferation, and a higher rate of node positivity.39,40 Furthermore, it is characterized by a unique molecular profile, aggressive nature, and lack of targeted therapies. There is an early peak of recurrence between year 1 and year 3 after diagnosis and it metastasizes to distinct locations involving viscera (lung, liver, and brain); it is less likely to spread to the bone.41 TNBC is associated with a poor overall prognosis; however, approximately half are not resistant to chemotherapy. Adjuvant chemotherapy has been shown to improve disease-free survival and overall survival; however, it is not possible to utilize targeted therapies at this time and research continues to find exploitable targets for this breast cancer type. Standard chemotherapy when administered in a neoadjuvant (NAC) fashion results in a pathological complete response (pCR) of 20% to 34% of TNBC.42–44 A pCR is associated with increased survival, and those who do not achieve this status are at high risk of disease recurrence.45 Despite initial chemo sensitivity and response to anthracycline and taxane-based therapies, a high risk of early relapse remains when the tumor is not completely eradicated. Recent studies have shown that the pCR may be improved in TNBC with the addition of platinum-based NAC; however, this continues to be a topic of debate in the treatment of TNBC.46,47 Additionally, it has been shown that cells deficient in BRCA1 and BRCA2 are exquisitely sensitive to poly (ADP ribose) polymerase (PARP) inhibition and several PARP inhibitors are being evaluated in clinical trials. Continued research efforts are underway to improve local and systemic control in this molecular subtype.
The NSABP B-18 trial showed that the administration of chemotherapy before surgery (neoadjuvant) did not improve disease-free survival or overall survival in comparison to administration after surgery (adjuvant).48 However, patients who achieve a pathologic complete response (pCR) following neoadjuvant chemotherapy have improved disease-free survival and overall survival rates compared to those without a pCR.49,50 Neoadjuvant chemotherapy is clearly indicated in patients who present with inoperable, locally advanced, and inflammatory breast cancer and recently in large operable breast cancer. The key advantages of neoadjuvant chemotherapy include the ability to enable breast-conserving surgery by downstaging patients prior to surgery and the ability to assess in vivo response to chemotherapy.51 In addition, pCR is an early surrogate in certain subgroups of breast cancer, triple negative and HER2/neu amplified, indicative of long-term outcome, including overall survival. The opportunity to shrink tumors before surgery allows inoperable tumors to be converted into operable, enables breast conserving surgery in those that would have otherwise required mastectomy, and allows for improved cosmetic outcomes with breast conservation. With systemic therapy, patients may have complete resolution of the tumor. Therefore, it is of utmost importance to properly stage patients prior to neoadjuvant therapy and to place a metallic clip under image guidance, marking the primary tumor site and allowing for identification at the time of surgical resection. Furthermore, systemic therapy upfront may eradicate microscopic disease in the regional nodes, decreasing the need for a complete axillary lymph node dissection at the time of surgery. However, for patients who had clinically positive nodes prior to neoadjuvant chemotherapy, axillary lymph node dissection remains the standard of care.
Furthermore, administration of chemotherapy prior to surgery provides important information about the in vivo response of the tumor to exposed agents and treats potential systemic disease without delay. Tumor biology can be better understood as the neoadjuvant approach that enables tumor tissue to be studied before, during, and after chemotherapy. By doing so, responses to the drugs can be evaluated in an expedited time frame. With adjuvant administration the endpoint is survival, whereas with neoadjuvant administration the endpoint is pathological response. More recently, a cited advantage of neoadjuvant chemotherapy has been that it provides a research platform for accelerated drug approval. An example of this is the recent U.S. Food and Drug Administration (FDA) approval of pertuzumab in patients with stage II and III HER2-positive breast cancer.
For locally advanced or inflammatory breast cancer, chemotherapy is routinely administered prior to surgery.52 By definition, locally advanced breast cancer (LABC) is a disease that extensively involves the lymphatics, chest wall, or both. There is no clinical evidence of distant metastasis but a high risk for micrometastasis and development of subsequent metastasis. Patients with locally advanced disease can have large primary tumors (>5 cm), chest wall or skin involvement, ulceration or satellite skin nodules, and inflammatory changes. Current management requires a multidisciplinary approach including surgery, radiation therapy, and systemic therapy.
Inflammatory breast cancer (IBC) is rare (less than 5%) but is one of the most aggressive and deadly subtypes of breast cancer.53 Patients with IBC have a twofold greater risk of dying from their disease than patients with LABC. IBC is manifested by erythema and edema of the breast and peau d’orange (Fig. 82-3) that develops within a 3-month time period as a result of lymphatic obstruction. The process is known as dermal lymphatic invasion caused by tumor emboli.54 Clinically, there may not be a palpable mass and there may be no abnormality seen on breast imaging other than skin thickening.55 IBC is a clinical diagnosis characterized by a rapid onset (weeks to months), the characteristic skin features, and a biopsy of the breast showing carcinoma; however, the main clinical symptoms and pathological characteristics are not always observed among patients with IBC, making it a difficult diagnosis.56 The incidence of IBC has increased over the last decade and occurs at higher rates among African American women.57 The median overall survival has remained low at an estimated 2.9 years; however, it has improved due to application of multimodality therapy beginning with standard anthracycline and taxane–based preoperative chemotherapy followed by mastectomy and radiation therapy.58 Single institutions that have applied a multimodality approach have seen modest improvements in outcomes over time.59,60
FIGURE 82-3:
Inflammatory breast cancer. A, B. Enlarged right breast with nipple retraction, edema, and erythema. C. Peau d’orange or an orange peel appearance of the skin involving the areola. (Reproduced with permission from National Cancer Institute at the National Institutes of Health. www.cancer.gov, 2012. Retrieved from Web 17 May 2012. http://www.cancer.gov/cancertopics/factsheet/Sites-Types/IBC.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






