Overview of Endoprosthetic Reconstruction
Martin M. Malawer
Kristen Kellar-Graney
BACKGROUND
Limb salvage—reconstruction following resection of malignant tumors of the extremities—has seen dramatic advances in a relatively brief period of time. The traditional surgical approach to the treatment of sarcoma, namely immediate amputation of the extremity, was advocated in the early 1960s and 1970s to ensure local control of disease.
Early pioneers in orthopaedic oncology worked diligently to define the optimal level of amputation and developed techniques to manage wounds of the pelvis and shoulder girdle following hind- or forequarter amputation. However, such aggressive surgical management failed to impact overall patient survival, with most patients dying of metastatic disease.
Only after the introduction of effective doxorubicin- and methotrexate-based chemotherapy protocols in the early 1970s could alternatives to amputation be considered. A handful of surgeons began to challenge the orthodoxy of amputation in children and adults with bone sarcomas. Marcove, Francis, and Enneking were among the pioneers who developed the rationale and basic techniques used in limb-sparing surgery. The former two surgeons were the first in the United States to develop endoprosthetic replacements for tumor patients.
Starting with a very few highly selected patients with extremity osteosarcoma, limb-sparing surgery now is a treatment option for most bone and soft tissue sarcomas not only of the extremities but of the pelvis and shoulder girdles as well.
Today, over 90% to 95% of tumor patients may be expected to undergo successful limb-sparing procedures when treated at a major center specializing in musculoskeletal oncology. This dramatic alteration in patient care required significant advances along many fronts, including the following:
Better understanding of tumor growth and metastasis
Determination of appropriate surgical margins
Use of effective induction (neoadjuvant or preoperative) chemotherapy
Development of improved approaches, preserving soft tissue vascularity
Deeper understanding of skeletal biomechanics
Advanced material engineering and manufacturing techniques
Development of inherently stable modular prostheses
The chapters in this section outline in specific detail many of the surgical approaches and techniques of oncologic resection and reconstruction currently used by leaders in the field of orthopaedic oncology. The importance of meticulous surgical technique cannot be overstated because this is vital to ensure an optimal oncologic and functional outcome for the patient. A successful limb-sparing surgery consists of three interdependent stages performed in sequence:
Tumor resection with appropriate oncologic margins
Reconstruction and stabilization of the involved bone and joints
Restoration of the soft tissue envelope for prosthetic coverage and function.
History of Endoprosthetic Reconstruction
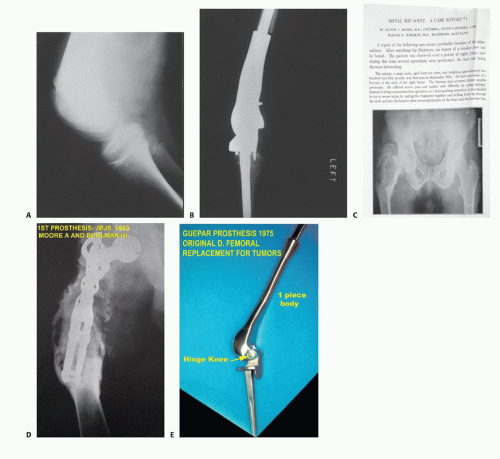
Austin Moore and Harold Bohlman,9 in 1943, were the first to publish an example of endoprosthetic reconstruction for a bone tumor, consisting of a custom-designed Vitallium proximal femur used for a patient with a giant cell tumor of bone.
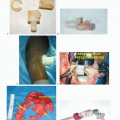
In the early 1970s, Francis and Marcove ushered in the current age of endoprosthetic reconstruction by developing prostheses to replace the distal femur and the entire femur for reconstruction following radical resection of osteosarcomas8 (FIG 1).
A major drawback for these custom implants quickly became evident: Each implant would take 6 to 12 weeks to manufacture, during which time the patient’s tumor could progress significantly. This led to the development of the concept of induction (initially called preoperative or neoadjuvant) chemotherapy, in which the newly proven drugs doxorubicin and methotrexate were administered during the interval between diagnosis and delivery of the manufactured custom implant.10 Both of these drugs had just been shown to have activity against bone sarcomas. Induction chemotherapy has since been adopted in the management of an increasingly large variety of other cancers.
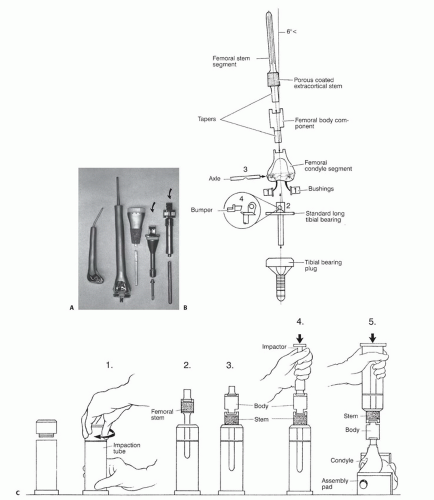
As the demand for endoprosthetic reconstruction grew, a wide variety of custom implants became available from a number of orthopaedic manufacturers. Many of these early implants, however, suffered from design flaws and errors in manufacturing, resulting in significant problems with implant failures (FIG 2A).
However, improved material and manufacturing techniques developed for the profitable and ever-expanding market for total joint replacements eventually were applied to these “mega” prostheses. The adoption of the rotating hinge for implants around the knee and bipolar heads for the hip followed successful use of these designs for total joint replacement. Although these advances significantly improved the performance of custom implants, problems with the time required for manufacturing and the lack of flexibility at the time of implantation hampered the widespread acceptance of custom endoprosthetic reconstruction.
Manufacturers responded to this problem by incorporating the concept of modularity, adapting concepts and designs from modular total hip and knee prostheses to develop
interchangeable and easily assembled endoprosthetic systems (FIG 2B,C). Although modularity increased the complexity of the mechanical construct and carried a risk of failure associated with the sum of all of the components, these potential problems were easily outweighed by significant benefits.
The primary advantage of a modular endoprosthesis is the system’s flexibility: The surgeon can concentrate on performing the best possible oncologic resection knowing that any changes in the preoperative plan can be accommodated by selecting those components that fit the patient’s anatomy and actual skeletal defect optimally.
Modular trial components allow the surgeon to mix and match pieces and test the reconstruction prior to selection and assembly of the actual final prosthesis.
Standardization of components permits the implant manufacturer to increase the level of quality control greatly while reducing the overall cost of manufacturing through economies of scale.
Modular systems reduce overall inventory and time to delivery while providing a large choice of prosthetic shapes and sizes.
Modular systems permit hospitals to maintain an on-site inventory that has allowed these systems to be available immediately as a backup option for selected nononcologic patients, such as those undergoing difficult joint revision surgery or patients with significant periarticular fractures.
A first-generation modular endoprosthetic system was the Howmedica Modular Replacement System (HMRS; Howmedica International, Limerick, Ireland), designed and manufactured in Europe. This system featured intramedullary cementless press-fit stems supported by external flanges and cortical transfixation screws while the knee mechanism
consisted of a simple hinge design. Although the system truly was modular, in clinical practice, the long-term outcomes were disappointing. Significant problems encountered with this device included aseptic stem loosening (osteolysis), substantial stress shielding with bone resorption, screw fracture and migration, and a polyethylene failure rate higher than 40% for the knee mechanism.4,6 Consequently, this system rarely was used in the United States.
An example of a second-generation modular system is the saddle endoprosthesis (Waldemar-Link Mark II Endo-Model Modular Saddle Prosthesis; Waldemar-Link, Hamburg, Germany; FIG 3A,B). This prosthesis, originally designed for the treatment of infected failed total hip replacements, was modified to allow for reconstruction of the hip following resection of the pelvis.
The unique feature of this system is the saddle itself, which is a U-shaped component that straddles the ilium, allowing motion in flexion-extension and abduction-adduction in the anteroposterior and lateral planes against the bone.
The saddle is attached with a rotating polyethylene-lined ring, increasing the degree of freedom and allowing for hip rotation. These are attached to a series of interchangeable modular bodies that, in turn, connect to a standard cemented femoral stem.
This device preserves limb length following resection of the periacetabulum (eg, type II pelvic resection, modified internal hemipelvectomy) while functioning like a total hip prosthesis. The clinical and functional results following saddle reconstruction of the pelvis with this system have been promising.1
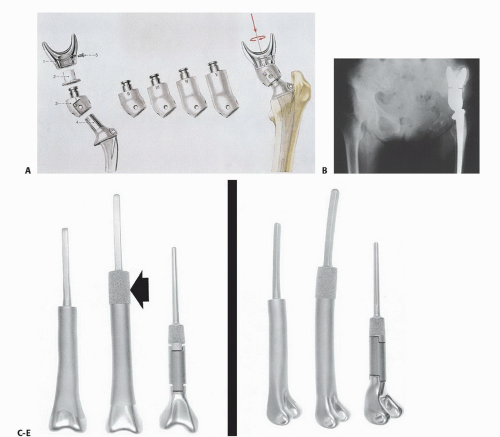
The first successful universal modular system was introduced in 1988 as the Modular Segmental Replacement System (MSRS; Howmedica, Inc., Rutherford, NJ), renamed the Modular Replacement System (MRS) and now available as
the updated Global Modular Replacement System (GMRS; Stryker/Howmedica, Inc., Mahwah, NJ; FIG 3C-E).
This system was designed to provide modular replacements for the proximal humerus, proximal femur, total femur, distal femur, and proximal tibia and has been instrumental in the widespread adoption of endoprosthetic reconstruction following segmental bone resection.
The growing popularity of endoprosthetic reconstruction has led to the introduction of similar modular systems from several orthopaedic manufacturers (eg, Orthopaedic Salvage System [Biomet, Warsaw, IN], Guardian Limb Salvage System [Wright Medical Technology, Arlington, TN]).
Current implant manufacturers still offer customized solutions for challenging anatomic issues. However, these custom implants often consist of a custom module mated to an existing modular system to ensure maximal flexibility.
TYPES OF ENDOPROSTHETIC RECONSTRUCTION
Specific anatomic examples of endoprosthetic reconstruction are discussed in the following paragraphs.
Hip
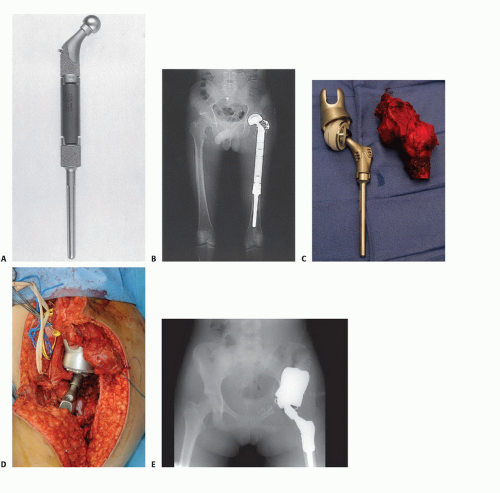
Tumors involving the proximal femur are extremely common and include both primary sarcomas and metastatic carcinomas. Replacement of the proximal femur (FIG 4) is readily accomplished following resection of a primary tumor or fracture through a subtrochanteric metastatic lesion. A bipolar hemiarthroplasty is used for the hip joint, with soft tissue reconstruction of the hip capsule to minimize the risk of dislocation.2 Reconstruction of the hip abductors is accomplished directly via laterally placed holes or loops or, if a portion of the trochanter was saved, by use of a trochanteric
claw with cerclage cables. Less common are resections of the entire hip joint (ie, type II pelvic resection and its modifications). This defect can be reconstructed with a saddle prosthesis or with the recently designed partial pelvic implants that attach to the remaining ilium. Stability is achieved by balancing the muscle tension between the medial iliopsoas and the lateral hip abductors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree