Fig. 24.1
Ultrasound of a one-month-old with antenatally diagnosed ovarian cyst, demonstrating layering of debris within a large thin walled cyst suggesting previous ovarian torsion. (Courtesy of Dr. Alistair Calder, Great Ormond Street Children’s Hospital, London, UK.)
The differential diagnosis of an intraabdominal cyst in a foetus or newborn includes renal and enteric duplication cysts and lymphatic malformations [15]. An absent ovary or a contralateral multicystic ovary increases the chances of the cyst being ovarian in origin. Enteric cysts have a thicker, two-layered wall on ultrasound examination.
Management
The criteria for intrauterine intervention are not well defined. Simple aspiration of larger cysts with a diameter of 5 cm or more [16, 17] may be followed by re-accumulation of fluid [9]. Massive cysts, which might obstruct labour, can be aspirated [11] before delivery. Bilateral ovarian cysts might be an indication for preterm delivery or intervention to prevent torsion [9].
Conservative management with serial ultrasound scans for asymptomatic unilateral neonatal cysts is recommended as most simple cysts will resolve spontaneously [18] and even some complex cysts containing debris will resolve leaving a follicular ovary with presumed function [5, 16]. Indications for intervention include a complex cyst with solid elements suggesting a neoplasm. In addition, cysts that do not resolve, and those with a diameter greater than 4 cm are at increased risk of torsion and intervention should be considered. Simple percutaneous aspiration is not recommended as a fatal outcome has been reported when a cyst of enteric origin was aspirated [19]. Reaccumulation of cyst fluid may occur because of stimulation by neonatal gonadotrophins, which peaks at 3–4 months post-natal age.
Post-operative Complications and Outcomes
Following surgery under a general anaesthetic, most newborns and infants make an uneventful recovery, establishing full feeds within 24–48 h. Reported wound complications include infection, mostly with staphylococcal organisms, port site hernias, and dehiscence with omental protrusion.
The ultimate functional outcome of a fetal ovarian cyst depends on its characterisation [8]. Simple cysts, which continue as simple cysts, have a good prognosis with 85% resolving spontaneously and leaving a functioning follicular ovary. However, complex cysts containing debris (see Fig. 24.1) have frequently undergone torsion and many will disappear on subsequent scans, with only 16% surviving as follicular ovaries. Approximately half of simple fetal ovarian cysts will become complex before birth [9], so that in neonates complex cysts outnumber simple cysts. Therefore, the prognosis for a fetal ovary containing a cyst of 2 cm or more in diameter is guarded, as the majority will become complex and either atrophy or be resected. For the rare case of bilateral ovarian cysts, preterm delivery or fetal intervention can be considered, although data from prospective trials is not available and the balance of the risks of intervention versus the risks of ovarian loss must be carefully considered.
Ovarian Cysts in Children
Aetiology and Incidence
The ovary in prepubertal girls is active with a low rate of maturation and involution of follicles [25] driven by intermittent release of pituitary gonadotrophins [26]. By ultrasound approximately 2% of girls under the age of 8 years will have small cysts, which may be bilateral. True precocious puberty with elevated levels of gonadotrophins may be the cause of multiple small cysts. However, cysts with a diameter over 1 cm may be hormonally active and associated with pseudoprecocious puberty [15]. In girls aged 2–5 years old, precocious puberty may be the presentation of the McCune–Albright Syndrome, with café-au-lait spots and polyostotic fibrous dysplasia occurring at a later age [25, 27].
With puberty the ovaries increase in size and multiple small cysts are common. The ultrasound appearance is often described as multicystic if the ovaries contain more than six follicles of greater than 4 mm diameter. These cysts result from the ovarian response to pulsatile gonadotrophin secretion [28]. A multicystic ovary should not be confused with a polycystic ovary, which is defined by the ultrasound appearance of 12 or more follicles measuring 2–9 mm in diameter. This can occur in 20% of healthy women but is also associated with the polycystic ovary syndrome [29]. This syndrome of ovarian dysfunction is managed medically by gynaecologists and endocrinologists without the need for surgical intervention.
The risk of malignancy in a prepubertal ovarian cyst is very low but cysts which persist and those with solid elements on imaging should be investigated and surgical excision considered (see the discussion of ovarian neoplasms later in the chapter).
Presentation and Management
Although most ovarian cysts are asymptomatic, pain can be triggered by torsion [30] or haemorrhage. Girls with signs of precocious puberty or uterine bleeding should undergo endocrine investigations and ovarian scanning. Tumour markers, serum α-fetoprotein (α-FP) and β-human chorionic gonadotrophin (β-HCG) should be measured and the ultrasound scan repeated after an interval of a few weeks. Most ovarian cysts in this age group will resolve spontaneously [31] but persistent, hormonally active, or symptomatic cysts should be excised by an ovarian sparing cystectomy preferably performed laparoscopically.
Outcome and Follow-Up
Histological examination of the excised tissue will confirm the diagnosis. Children with signs of precocious puberty or abnormal hormone production should be followed by a paediatric endocrinologist.
Ovarian Cysts in Adolescents
Aetiology and Incidence
Follicular cysts are common in adolescents. They are fluid-filled simple cysts, usually 2–3 cm in diameter that arise in the first half of the menstrual cycle. They and resolve in the second half of the cycle. However, if ovulation does not occur, they can grow to a very large volume. Corpus luteal cysts develop in the second half of the menstrual cycle and secrete progesterone. They can grow up to 6 cm in diameter before rupturing.
In addition to physiologic follicular and corpus luteum cysts the differential diagnosis of an ovarian cyst in an adolescent includes neoplasms, ectopic pregnancy, endometriosis, tubal anomalies, and the sequelae of sexually transmitted infections [25].
Presentation and Management
Irregular menses and pain secondary to rupture and haemorrhage are the common presentations. A palpable mass was reported in 14% of girls with ovarian cysts in one series [32]. Most follicular and corpus luteal cysts will resolve over 4–5 weeks, although larger cysts with diameters up to 8 cm may take 3 months to resolve.
The indications for surgical intervention include persistence of the cyst beyond 3 months, the risk of ovarian torsion (Fig. 24.2), symptoms that do not resolve, and solid elements on imaging suggestive of a neoplasm. Preservation of viable ovarian tissue is of paramount importance and consultation with an experienced adolescent gynaecologist may reduce the number of unnecessary oophorectomies [33]. Laparoscopy confirms the ovarian origin of the lesion. Since simple aspiration of the cyst leads to a high incidence of recurrence [34], cysts are best managed with fenestration of the cyst or cystectomy.
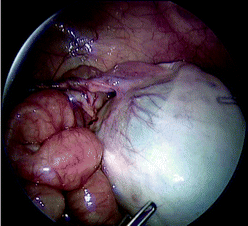

Fig. 24.2
Laparoscopic view of a neonatal ovarian cyst with torsion of the long ovarian pedicle
Torsion of the ovary is managed initially by derotation to assess tissue viability. Ischaemic and damaged ovarian tissue has remarkable potential for recovery, which can be assessed at a second laparoscopy delayed 2–3 weeks after derotation [35]. Definitive surgery for the ovarian cyst can be safely performed at the second laparoscopy. Fixation of the contralateral normal ovary is controversial, as the prevention of possible but rare future torsion must be balanced against adhesion formation impairing fertility [36, 37].
Endometriosis can occur in adolescents as well as in adults [38]. Endometriomas are blood-filled cysts arising in functioning endometrium within the ovary. The presence of altered blood gives a typical “chocolate cyst” appearance on ultrasound scanning and at surgery. The clinical presentation may be with acute pain secondary to ovarian torsion or haemorrhage but a more frequent presentation is with increasingly painful menstrual periods in an adolescent girl. The diagnosis is suggested by the appearances on ultrasound and confirmed at laparoscopy. The treatment is laparoscopic cystectomy and clearance of other pelvic endometrial deposits. If the endometriosis is extensive, a second laparoscopic procedure may be required after a 3 month course of hormonal suppression with gonadotrophin releasing hormone. Surgical intervention has been shown to relieve symptoms in the short term but there is little data on long-term outcomes and future fertility [39, 40].
Outcome
Histology of excised tissue will confirm the diagnosis. For functional cysts, fenestration has a low recurrence rate with the best chance of preserving ovarian tissue [3]. Cystectomy is required if a neoplastic cyst is suspected. Very large cysts and those with elevated tumour markers can be managed with open surgery through a Pfannenstiel incision. Ovarian preserving resections are the initial procedure of choice because malignant tumours are rare in cystic disease of the ovaries.
Ovarian Tumours
Incidence and Classification
The interest in ovarian tumours is related to their great variety rather than their incidence, which is only approximately 2–3 girls per 100,000 population annually [18, 41]. A tertiary paediatric surgical centre may only see a few patients a year with ovarian tumours, three-quarters of which are benign. Malignant ovarian tumours represent only 1% of childhood cancer [42]. However, there is a larger variety of tumours arising from the ovary than from any other organ (Table 24.1). Tumours arise from the three embryological cell lines found in the ovary, with germ cell tumours being the most common, accounting for 75% of ovarian neoplasms, followed by sex cord–stromal tumours, 15%, and finally, epithelial tumours, which usually present in adolescents, making up around 10% [43–45]. The very rare variants of germ cell tumours and metastatic tumours occurring in the ovary will not be discussed. The aetiology of these tumours is largely unknown, although gonadoblastomas, are associated with chromosome anomalies and the WT1 mutation of Frasier syndrome [46, 47].
Table 24.1
Classification of ovarian tumours in children
Germ cell tumours (75%) |
∙ Dysgerminomas ∙ Yolk sac tumours (endodermal sinus tumour) ∙ Teratomas – Mature (Dermoids) – Immature – Embryonic ∙ Choriocarcinoma ∙ Gonadoblastoma ∙ Embryonal carcinoma ∙ Mixed germ cell tumours |
Sex cord–stromal tumours (15%) |
∙ Thecomas ∙ Fibromas ∙ Juvenile granulosa cell tumours ∙ Sertoli–Leydig cell tumours (Arrhenoblastomas) |
Epithelial tumours (10%) |
∙ Serous – Benign – Borderline – Malignant ∙ Mucinous – Benign – Borderline – Malignant ∙ Endometrioid |
Miscellaneous |
∙ Lymphoma ∙ Leukaemia ∙ Metastatic tumours – Neuroblastoma – Rhabdomyosarcoma – Nephroblastoma |
Pathology of Benign Ovarian Tumours
Thecomas occur rarely in the second decade, with some producing oestrogens and presenting with precocious puberty [48] and others containing androgen-producing lutein cells leading to virilisation [49]. These tumours may be calcified.
Fibromas are rare in childhood and can grow to a large size and be associated with ascites and pleural effusions (Meigs Syndrome). Fibromas have also been described in children with the basal cell nevus syndrome [50].
Gonadoblastomas contain mixed germ cell and sex cord–stromal cell elements and are frequently bilateral. They most often occur in phenotypically female patients with 46 XY or an XY mosaic karyotype [50]. Although benign, they arise in dysgenetic gonads and are associated with malignant ovarian germ cell tumours. Frasier syndrome is the association of nephropathy and gonadoblastoma in a phenotypic female with XY chromosomes and is associated with the WT1 gene [46].
Mature cystic teratomas (MCTs), also known as ovarian dermoid cysts, are the most frequently occurring ovarian tumours accounting for approximately 40% of all ovarian neoplasms in children. The cells are mostly diploid and arise in primordial germ cells which have entered meiosis [42]. All three germ layers may be present, with ectodermal elements predominating including teeth and hair (Fig. 24.3a and b). In children, these are benign tumours containing both solid tissue and cystic areas, with 10% occurring bilaterally. Late recurrent malignant germ cell tumours have been described in young adult women with a previously excised mature cystic teratoma [51].
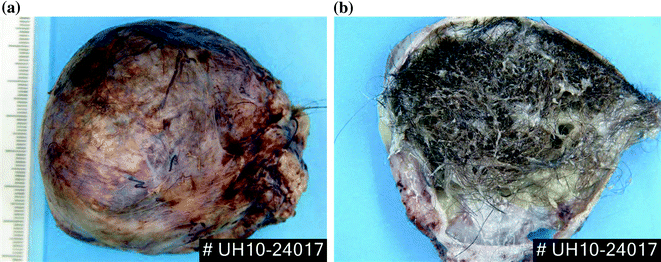

Fig. 24.3
An excised ovarian dermoid cyst (mature teratoma) a external surface b transected to demonstrate hair in the cyst. (Courtesy of Dr. Elizabeth Benjamin, University College London Hospital, London, UK.)
Immature teratomas are less common than MCTs and contain immature neuroectodermal elements. A careful histological search has to be made within these tumours for yolk sac elements, which are potentially malignant.
Gliomatosis peritonei are nodules of glial tissue sited on the peritoneum or omentum and have been described in patients with immature ovarian teratomas. Studies suggest that these are metaplastic cells arising from peritoneal stem cells and not seeded from the ovarian tumour [52].
Pathology of Malignant Ovarian Tumours
Dysgerminomas represent 11% of paediatric ovarian tumours, making them the most common malignant ovarian tumour in children. They are analogous to testicular seminomas. They are solid tumours and are bilateral in up to 15% of patients [53]. Microscopically they consist of epithelioid cells and mature lymphocytes. In addition, multinucleated cells and syncytiotrophoblastic giant cells may be present and produce β-HCG [54]. Dysgerminomas are found in mixed germ cell tumours and can arise with gonadoblastomas in dysgenetic gonads. Dysgerminomas usually present as localised stage 1 disease [55] but metastases can be found in pelvic and retroperitoneal lymph nodes, with rare haematogenous spread to the liver, lungs and bone. Serum lactic dehydrogenase is a useful tumour marker for many of these tumours [56].
Yolk sac tumours or endodermal sinus tumours are the second most common malignant ovarian tumour in children, most often arising in post-menarchal teenage girls but also rarely occurring in younger girls [57]. These solid tumours contain undifferentiated embryonic cells which produce α-fetoprotein [58]. These are aggressive tumours which spread locally in the peritoneal cavity, via the lymphatics to retroperitoneal and pelvic lymph nodes, and via the bloodstream to the liver, lungs and brain [59].
Mixed germ cell tumours make up one-third of malignant germ cell tumours and contain more than one neoplastic germ cell element, mostly dysgerminomas, yolk sac tumours, immature teratomas, and the rarer germ cell malignancies. Malignant germ cell tumours may be aneuploid and may exhibit the isochromosome 12p [60, 61]. They can produce α-fetoprotein or β-HCG [57].
Juvenile granulosa cell tumours are the most common of the stromal sex cord tumours and account for up to 10% of ovarian malignant tumours in children and adolescents [62]. They have cystic and solid elements and secrete oestrogens [63]. Mixed granulosa and thecal cell tumours also occur [64]. They are hormonally active and usually present at an early stage before spread has occurred outside the ovary. Adults with the Peutz–Jeghers Syndrome have an increased risk of developing sex cord–stromal tumours [65].
Sertoli–Leydig tumours or arrhenoblastomas are rare malignant tumours of the ovary with the majority presenting before the age of 20 years [66] and most will produce androgens [49]. Production of α-fetoprotein has also been reported [67]. They have cystic and solid elements and are usually localised to the ovary at presentation.
Epithelial cell tumours are much less common in children and adolescents compared to adults and are classified into serous and mucinous subtypes. The majority are benign or borderline with low malignant potential [68]. Borderline epithelial tumours have varying nuclear atypia but lack stromal invasion and are more common in adolescents [45, 69]. Malignant adenocarcinomas are very rare in adolescents and children [57, 70, 71]. Borderline tumours spread locally in the pelvis and have a high incidence of bilaterality. Malignant epithelial tumours spread in the peritoneal cavity to retroperitoneal and pelvic lymph nodes and via the bloodstream to liver and lungs.
Lymphomas are rare ovarian tumours in Europe and America but are the most commonly described ovarian tumour in West African children [72].
Presentation of Ovarian Tumours
The majority of ovarian tumours will present with symptoms or clinical signs while a minority will be found incidentally on imaging at laparotomy or at laparoscopy [18]. Pain, usually described by children in the lower abdomen, is the most common symptom. Acute severe pain suggests ovarian torsion, while recurrent or chronic pain of less severity may be the result of haemorrhage into or rupture of the tumour [73]. Lower abdominal distension may be the only complaint at presentation. A palpable mass may be detected arising from the pelvis or in the lower abdomen, indicating a larger tumour with a diameter greater than 7 cm and a higher risk of malignancy [74]. Hormonally active ovarian tumours are rare and present with signs of precocious puberty, vaginal bleeding, or virilisation.
Investigations of Ovarian Tumours
Imaging starts with ultrasound scanning (Fig. 24.4) and colour flow Doppler to assess blood flow to the lesion and ovary but ultrasound scanning does not always distinguish between benign and malignant lesions. However, lesions with an irregular outline, significant solid elements and a diameter greater than 7.5 cm have a higher risk of malignancy [74]. A smooth capsule and predominantly cystic structure increases the chances that the lesion is localised and benign [75].
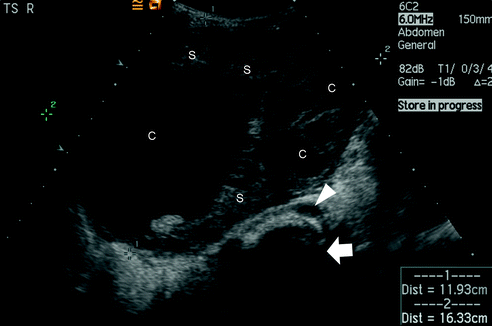

Fig. 24.4
Transverse abdominal ultrasound scan in a six year old girl presenting with an abdominopelvic mass and premature thelarche. The large mass contains cystic (c) and solid (s) elements. The arrow points to the spine and the arrowhead to the abdominal aorta. (Courtesy of Dr. Alistair Calder, Consultant Radiologist at Great Ormond Street Children’s Hospital, London, UK.)
MRI scanning provides better anatomical detail with differentiation between uterine and ovarian lesions [76–78]. Pathologically enlarged lymph nodes, ascites, and liver metastases may also be identified on magnetic resonance imaging. If vascular detail is required, more information will be gained from CT scanning with vascular contrast but at the biological cost of additional irradiation. CT scans of the chest will identify lung metastases and would be performed when staging the rare malignant ovarian tumours.
Tumour markers are useful but cannot distinguish between malignant and benign tumours [79]. Tumour markers may be elevated in benign and immature germ cell tumours but raised levels should always increase the suspicion of a malignant neoplasm. Table 24.2 indicates the tumour markers seen in ovarian tumours.
Table 24.2
Tumour markers
Tumour | Markers |
|---|---|
Teratoma | α-FP |
Yolk sac tumour | α-FP |
Dysgerminoma | β-HCG and LDH-1 |
Choriocarcinoma | β-HCG |
Embryonal carcinoma | α-FP and β-HCG |
Sertoli–Leydig tumour | α-FP |
Epithelial tumours | CA-125 |
Girls with precocious puberty or signs of virilisation should have endocrine investigations, including pituitary gonadotrophins, oestradiol, progesterone, and testosterone levels. Ovarian cysts and tumours are uncommon causes of precocious puberty.
Surgery for Ovarian Tumours
Most ovarian tumours are managed by surgical excision with preservation of ovarian tissue whenever possible [80]. A staging procedure is appropriate when malignancy is suspected. Staging for germ cell tumours (Table 24.3) is less invasive as no additional information is gained from sampling peritoneal tissue and lymph nodes which are palpably and macroscopically normal [81]. However, when malignant epithelial or sex cord–stromal tumours are suspected, sampling should include peritoneal, omental, and lymph node biopsies since metastatic spread tissue may be macroscopically and palpably normal (Table 24.4) [82, 83].
Table 24.3
Children’s Oncology Group (COG) staging for ovarian germ cell tumours
Stage | Description |
|---|---|
Ia | Limited to one ovary |
Ib | Limited to both ovaries |
II | Microscopic residual or positive lymph nodes <2 cm diam. |
Peritoneal evaluation and washings negative | |
± Tumour markers | |
III | Lymph node involvement ≥2 cm diam. |
Gross residual tumour or biopsy of tumour only
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|