Fig. 23.1
Development of the ovary. Copyright JE Dietrich and NA Sokkary
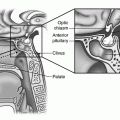
The ligament that helps to guide each ovary into position is known as the gubernaculum, which in its primitive form is a cord of mesenchyme connected to the lower medial pole of the gonad. The gubernaculum then attaches to the uterus and ultimately becomes the fibrous bands of the utero-ovarian (or meso-ovarian or ovarian) ligament, which attaches the ovary to the lateral surface of the uterus, and the round ligament, which extends from the uterine horn (the junction of the uterus and the fallopian tube) through the internal inguinal ring to the mons pubis (Fig. 23.2) [4]. Another primitive ligament at the lateral-superior aspect of the gonad becomes the infundibulopelvic ligament or suspensory ligament of the ovary (Fig. 23.2). Ultimately, a normally positioned ovary lies below the pelvic brim within the “true” pelvis, suspended between the utero-ovarian and infundibulopelvic ligaments [4]. It is important to note that the process of ovarian development is completely independent of the Müllerian development of uterus, fallopian tubes, and vagina [1, 2].
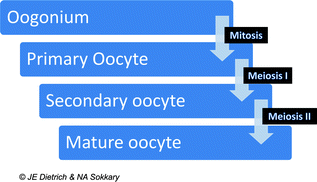

Fig. 23.2
Stages of oogonial development. Copyright JE Dietrich and NA Sokkary
Embryology of the Ovum
The development of a human egg, or ovum, capable of being fertilized is the result of a complex process that begins early in embryogenesis and proceeds through a series of orderly stages through sexual maturity. Primitive germ cells originate from the yolk sac endoderm and migrate to the gonadal ridges (Fig. 23.1). The gonadal ridges form primitive sex cords made of up of both ectoderm and mesenchyme [1]. Primitive germ cells in the sex cords give rise to oogonia (plural of oogonium) by 6–7 weeks gestational age [1]. With repeated divisions the number of oogonia peaks around 16–20 weeks gestational age when 6–7 million may be present. After this peak, a slow process of apoptosis of the oogonia ensues. Oogonia that do not undergo apoptosis are transformed to the 1–2 million oocytes (immature ova) that are present at birth. Through the specialized process of cell division called meiosis the diploid oogonium is transformed to a haploid mature oocyte (Fig. 23.3). The development of an oocyte occurs within a capsule of somatic cells in the cortex of the ovary known as a follicle. Each follicle typically contains only one oocyte [2]. Through a process of slow apoptosis, the number of oocytes decreases throughout life. By the reproductive years only 300,000–500,000 oocytes remain and of these, only 400–500 will become mature ovulatory follicles [1, 3].
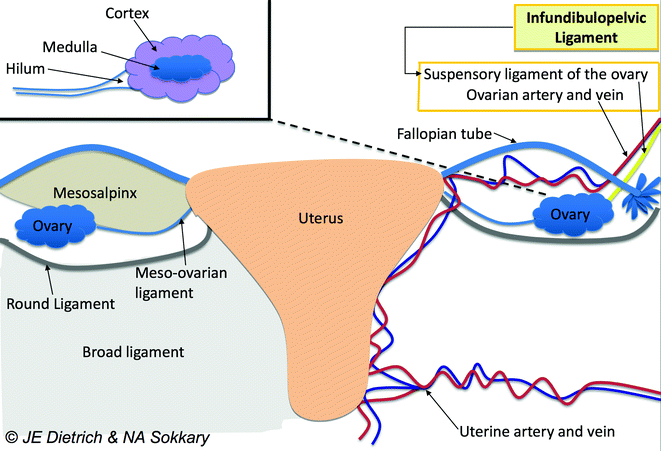

Fig. 23.3
Anatomy of the ovary. Copyright JE Dietrich and NA Sokkary
The process of oocyte development begins prenatally with duplication of genetic material and formation of the primary oocyte that then undergoes begins meiosis I. The process of meiosis I is halted in prophase and primary oocytes remain in this state until menarche. During meiosis I there is recombination of genetic material maternal between homologous chromosomes which increases genetic diversity. With menstruation meiosis I is completed and the genetic material of the primary oocyte is divided resulting in a secondary oocyte, and a polar body (a nonfunctional primordial entity) and meiosis II begins [1, 3]. The secondary oocyte remains frozen in meiosis II until fertilization when a mature ovum and another polar body is produced.
A primary oocyte is a complex structure made up of yolk, the germinal vesicle (the large nucleus of the primary oocyte before meiosis completed) and the germinal spot (the nucleolysis of the oocyte) [2]. Although made up of many complex parts, human oocytes are relatively fragile, measuring only 0.2 mm in diameter. Each has protective layers including a thick, transparent envelope known as the zona pellucida and an outer layer called the corona radiata [1, 2].
Anatomy of the Ovary and Adnexa
The ovaries, fallopian tubes, and broad ligament complexes make up the adnexa of the uterus. The ovary is normally located just posterior to the fallopian tube on the pelvic sidewall. The ovary is in close proximity to the fallopian tube, and is, in fact, connects the tube by a small muscular complex, known as the fimbria ovarica [5]. The position of the tube near the ovary allows “sweeping” motions of the fimbria of the fallopian tube to collect mature ova from the ovarian surfaces.
The blood supply to the ovary on each side is redundant with a continuous arcade of vessels to supply and support the adnexal structures [5]. Specifically, the ovarian arteries (originating from the renal artery on the left and the aorta on the right) located within the infundibulopelvic ligaments, supply the lateral aspect of the ovary, while Sampson’s artery, within the round ligament, gives rise to arterial branches within the broad ligament and mesosalpinx.
The adnexa are covered by a series of specialized peritoneal folds. Closest to the ovary is a layer known as the mesovarium, which ultimately connects to the mesosalpinx. The continuity of the peritoneal folding (described previously in Embryology of the Ovary) means that the entire adnexa can be moved when one aspect is moved (Fig. 23.2) [5].
In a neonate, the ovary is typically 1 cm in diameter and weighs 250–350 mg. Commonly, the right ovary is slightly larger than the left ovary [1, 5]. From the neonatal period to the reproductive years, the ovary will ultimately increase its weight by 10 times. The ovary consists of 3 major portions—the outer cortex, the central medulla, and the ovarian hilum (Fig. 23.2) [1, 5]. The hilum is the point of attachment of the ovary to the mesovarium, the folds of peritoneum that tether the ovary to the body wall [1, 5]. The hilum contains blood vessels, nerves, and hilus cells. The hilar cells are the origin of certain types of ovarian tumors, such as Sertoli or Leydig cell tumors [5]. The outer cortex consists of the tunica albuginea, made up of cuboidal and germinal epithelium. The cortex of the ovary is responsible for follicle formation and is responsive to follicle stimulating hormone (FSH). The supporting network or stromal support tissues within the cortex have the ability to respond to luteinizing hormone (LH) or human chorionic gonadotropin (hCG). The central medulla is largely comprised of mesonephric cells without hormonally sensitive components [1, 5].
Physiology of the Ovary
The ovary has two main physiologic responsibilities: (1) the release of oocytes and (2) the production of steroid hormones [1]. Although Hippocrates, Soranus, and Galen all provided early descriptions of the ovary, it was not until the 1500s when Andreas Vesalius, a Professor of Surgery at the University of Padua, described ovarian follicles that we began to understand ovarian function [1, 3]. Ovarian function is a cyclical process driven by the hypothalamic-pituitary axis and is not static, but changes throughout a female’s life, from the time of fetal development to the postmenopausal years [1, 3].
At 18–20 weeks gestation, cells in ovarian cortex develop receptors which allow the ovary to respond to the pituitary hormones FSH and LH [1, 3]. At this time, the female fetus may develop transient ovarian cysts. The hormonal environment that allows formation and persistence of these cysts usually lasts into the neonatal period and for up to 6 months of neonatal life. A hormonal nadir is usually reached by 6 months of age and no later than 2 years of age and hormone levels do not rise again until 4–10 years of age, when hypothalamic-pituitary hormones are upregulated once again [3].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree