Fig. 10.1
Incidence and mortality age standardized rate of ovarian cancer according to United Nations world regions in 2012 (United Nations 2014)
There are several factors that increase the risk of developing ovarian cancer (Table 10.1). More than a quarter of ovarian cancers are diagnosed in women aged(Vo and Carney 2007) 75 and over. While epithelial ovarian cancers, the most common type of ovarian cancers are more prevalent in post-menopausal women, germ cell tumours are common in younger women under 40 years. Smoking is specifically associated with mucinous ovarian tumours. Nulliparity is an independent risk factor for ovarian cancer and fertility-inducing drugs do not influence ovarian cancer in a recent Cochrane review. However, there is an association of borderline ovarian tumours in women who receive in-vitro fertilization. Polycystic ovary syndrome, obesity and endometriosis are associated with a small increased risk of ovarian cancer.
Table 10.1
Risk factor for ovarian cancer
Risk factors |
|---|
Age |
Genetics |
Nulliparity |
Ovulation induction |
Smoking |
Occupational exposure |
Obesity |
Polycystic ovary syndrome |
Endometriosis |
Occupational exposure |
10.2 Risk Reduction
Several factors offer risk reduction of ovarian cancer. Pregnancy offers protection for ovarian cancer since there is a break to ovulation, thereby reducing the incessant breach of ovarian capsule. Relative risk of ovarian cancer is reduced by 20% for every 5 years use of OCPs and the risk is almost halved by 15 years use of the pills. The benefit of using oral contraceptive pills is seen even after 30 years of stopping the pills – and this is ascribed not only to anovulation, but also due to stabilizing effect of progesterone. Likewise, breast- feeding offers protection.
Increasingly evidence is emerging that ovarian cancers arise from the fallopian tube rather than de-novo. Hence tubal ligation or prophylactic salpingectomy in high-risk women (e.g. women with BRCA mutations) reduces the lifetime risk of ovarian cancer. In a meta-analysis, the risk reduction was 34% and when tubal ligation was carried out in women with BRCA mutations, the risk reduction could be as high as 60%. A history of OCP use and tubal ligation together offer a protection as high as 70%. Hysterectomy can be associated with ‘sympathetic’ ovarian atrophy contributing to risk reduction of almost 34%.
10.3 Genetic Predisposition
Ovarian cancers are mostly sporadic in nature (90%) while the remainder 10% has a genetic predisposition. Family history is quite important with the lifetime risk of developing ovarian cancer of 4–5% if one family member is affected and around 7% if two family members are affected. Hereditary ovarian cancer syndrome defined as two first-degree family members being affected with epithelial ovarian cancers carries a risk of 13–50% of developing ovarian cancer.
Genetic mutations associated with ovarian cancer are listed below in Table 10.2:
Table 10.2
Genetic mutations associated with ovarian cancer
Genetic predisposition | Defective gene | Risk of ovarian cancer |
|---|---|---|
Breast cancer associated gene mutations (BRCA) | BRCA1 – Chromosome 17 BRCA2 – Chromosome 13 | 35–46% (Risch et al. 2006) 13–23% |
Lynch syndrome | MLH1, MSH2, MSH6, PMS2 | 3–14% (Barrow et al. 2009) |
Peutz Jeugher syndrome | STK11 gene – Chromosome 19 | 20% |
Ovarian cancers due to a genetic mutation present around 10 years earlier than in patients with sporadic ovarian cancers. BRCA associated ovarian tumours have a unique clinic-pathological presentation. They are more likely serous cancers, and unlikely borderline or mucinous. They present with a higher grade and advanced stages (III/IV) when compared to sporadic ovarian cancers. In spite of these features, they hold a better prognosis, and this is attributed to better chemo-responsiveness. Studies (Bolton et al. 2012) have shown that stage, grade and histology-adjusted 5 year all-cause mortality was 45% in BRCA1 carriers versus 47% in non-carriers (hazard ratio [HR] 0.73, 95% CI 0.64–0.84) and 36% versus 47% for BRCA2 carriers versus non-carriers (HR0.49, 95% CI 0.39–0.61).
Risk reducing surgery – bilateral salpingo-oophorectomy can be offered to women with a known genetic mutation or strong family history. This has to be undertaken with active input from clinical geneticists as well as taking into consideration issues around fertility and premature menopause. Occult malignancy may be found in such cases in almost 4–8% women, and at the of 45, the chances can be as high as 20%.
10.4 Pathology
Primary ovarian cancers can be broadly classified as epithelial, germ cell and stromal tumours (Table 10.3). Almost 10 years ago, a new classification was proposed that separated ovarian cancers into type I and II tumours. Type I tumours include endometrioid, mucinous or clear cell types, and they are low grade and harbour mutations in BRAF, KRAS, and PTEN genes with microsatellite instability. Type II tumours include high-grade serous cancer and carcinosarcoma, which frequently contain mutations in p53, BRCA1, and BRCA2 genes. Although the latter are thought to arise from ovarian surface epithelium, there is emerging evidence that the precursor of the cancer arises from the fallopian tube (fimbria). This explains the risk reduction seen with salpingectomy/sterilization for women with increased risk of ovarian cancer.
Table 10.3
Ovarian tumour types
Origin | Surface epithelial cells (common epithelial tumors) | Germ cell | Sex cord-stroma | Metastasis to ovary |
|---|---|---|---|---|
Frequency | 65–70% | 15–20% | 5–10% | 5% |
Age group affected | 20 + years | 0–25 + years | All ages | Variable |
Types | Serous Mucinous Endometrioid Clear cell Brenner Unclassified | Teratoma Dysgerminoma Endodermal sinus Choriocarcinoma | Fibroma Granulosa-theca cell Sertoli-Leydig cell |
10.5 Presentation and Investigations
Often symptoms are vague in the initial stages and manifest either as pressure symptoms or gastro-intestinal disturbances. To trigger an earlier diagnosis of ovarian cancer, the National Institute of Clinical Excellence (CG122, NICE guideline, 2011) has suggested performing a CA-125 as first line if women (especially age > 50) have persistent symptoms (> 12 times a month) such as:
Abdominal bloating
Pelvic/abdominal discomfort
Early satiety
Increased urinary frequency/urgency
CA-125 is also recommended if a new diagnosis of irritable bowel syndrome is made in women aged > 50 years. Wherein the CA-125 is raised, an USS pelvis needs to be organized to assess ovaries.
10.5.1 Risk of Malignancy Index (RMI)
Risk of malignancy index (RMI) (Jacobs et al. 1990) calculation helps to assess the nature of an ovarian mass. RMI is calculated as follow:
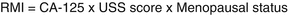
The CA-125 value is taken as whole; USS score is either 1 or 3 depending on one or more features found on USS (bilateral ovarian mass, presence of ascites, presence of solid areas, multilocular cysts or evidence of metastases); Score for menopausal status is 1 for premenopausal and 3 for postmenopausal women. Risk of cancer with a low RMI (< 25) is less than 3% while the risk is as high as 75% with a high RMI > 250.
This is quite useful to counsel the patient appropriately before undertaking a major surgery and at the same time, organize the necessary team to perform the surgery. In the UK, where cancer care is centralized for ovarian cancers, a high RMI merits patient referral to a tertiary centre for surgery, rather than management in a cancer unit. A recent systematic review showed the pooled sensitivities and specificities of an RMI I score of 200 in the detection of ovarian malignancies to be: sensitivity 78% (95% CI 71–85%), specificity 87% (95% CI 83–91%).
There are few drawbacks using the RMI. It is heavily reliant on CA-125 levels and it may be elevated in some benign conditions like endometriosis, benign ovarian tumours (eg Meigs’ syndrome), pelvic inflammatory disease, pregnancy, and menstruation leading to an elevated RMI. Also, in about 50% of early stage epithelial ovarian cancers, CA-125 may not be elevated leading to false reassurance (Dodge et al. 2012). CA-125 can be elevated in other non-gynaecological conditions including pancreatitis, pleural and pericardial disease, diverticulosis, leiomyoma and ascites. There is emerging evidence for new tumour markers such as Human Epididymis protein (HE4) (Ferraro et al. 2013) might be superior to CA-125 in detection of ovarian cancer, but more studies are needed before the research translates into clinical practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree