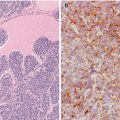
Fig. 6.1
Solid cell nests (SCNs) of the human thyroid. SCNs appear as cellular clusters with a lobulated configuration within the interstitium of the thyroid gland (a–c). They are usually solid squamoid nests (a) mainly composed of main cells with a minor proportion of C cells. Sometimes, however, SCNs are totally cystic (b) or combine solid, cystic and/or papillary architecture (c)
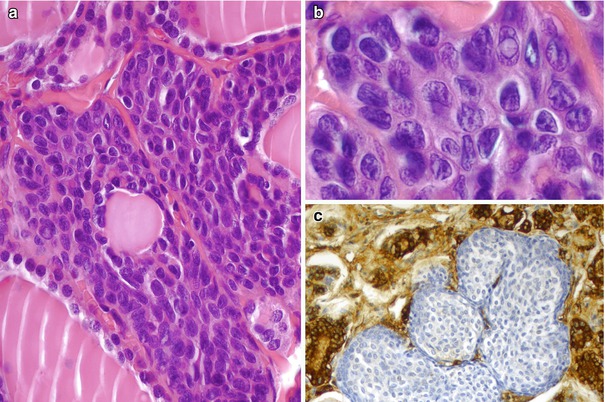
Fig. 6.2
Nuclear features in solid cell nests (SCNs). The nuclei of main cells of SCNs are round to oval with grooving and frequent overlapping (a), but intranuclear pseudoinclusions are very rare (b). Although these vestigial structures can be misinterpreted as papillary thyroid microcarcinoma, they are negative for thyroglobulin (c) and thyroid transcription factor-1 (TTF-1)
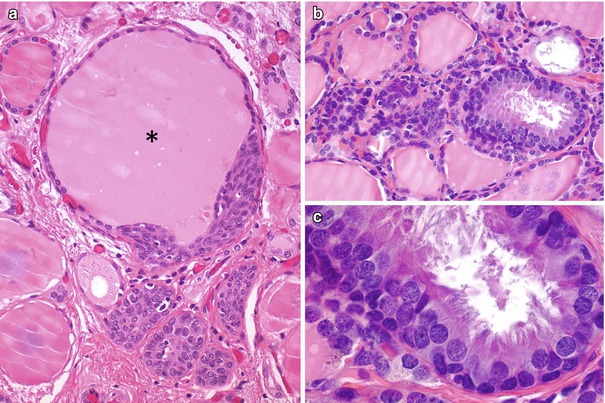
Fig. 6.3
Mixed follicles and ciliated cells in solid cell nests (SCNs). Occasional SCNs are connected to thyroid follicular cells to form mixed follicles (asterisk) (a). Approximately 20 percent of the cystic structures in SCNs are lined by ciliated columnar cells (b, c)
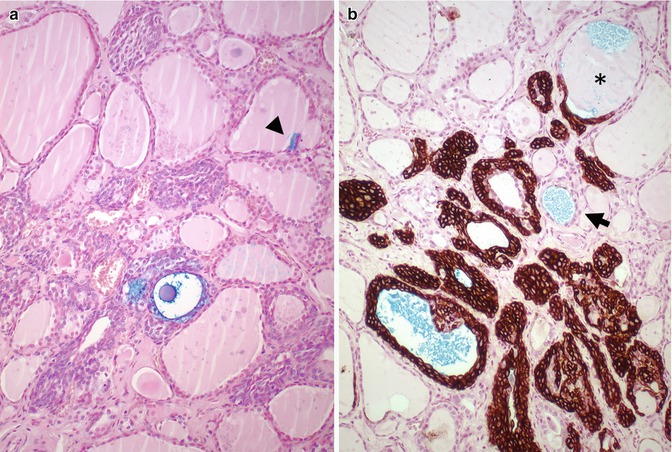
Fig. 6.4
Thyroid follicles with mucins in solid cell nests (SCNs). The SCNs may contain isolated cells (arrowhead) and small cysts with intraluminal accumulation of acidic mucins demonstrable with the Alcian blue stain (a, b). In the human thyroid gland, follicles with acid mucins (arrow) are almost always confined to the sections that also contain SCNs (b). A mixed follicle containing acidic mucins and colloid (asterisks) can be spotted easily in the upper part of the photograph because of the positivity of main cells for keratins (clone 34βE12) (b). It has been suggested that the histogenesis of some mucin-producing thyroid tumours could be linked to “ultimobranchial” thyroid follicles with acid mucins
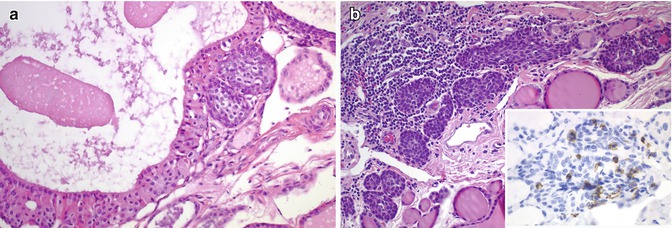
Fig. 6.5
Oncocytic change and lymphocytic infiltration in solid cell nests (SCNs). It has been reported the existence of rare cases of main cell of SCNs with oncocytic (Hürthle cell) changes (a). This finding indicates that SCNs may acquire mitochondrial alterations similar to those seen in follicular and C cells, as well as in their respective tumours (a). A variable proportion of scattered T-lymphocytes positive for CD5 (inset) are usually associated with SCNs, probably due to a common embryologic origin of SCNs and thymus (b)
Ultrastructurally, the main cells of SCNs have many desmosomes and hemidesmosomes at the cell surface. Filaments are the only cytoplasmic evident organelles while other are poorly developed, as it happens in stem cells. Main cells are positive for high and low molecular weight keratins, p63 , p40, galectin-3 , Bcl-2 and carcinoembryonic antigen (CEA) (Fig. 6.6), and negative for TTF-1, PAX8, thyroglobulin, calcitonin, chromogranin and synaptophysin . Calcitonin and neuroendocrine pan-markers are only positive in the associated C cells (Fig. 6.7).
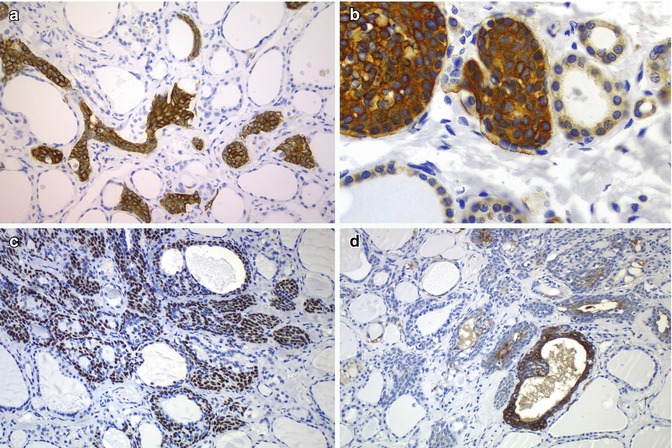
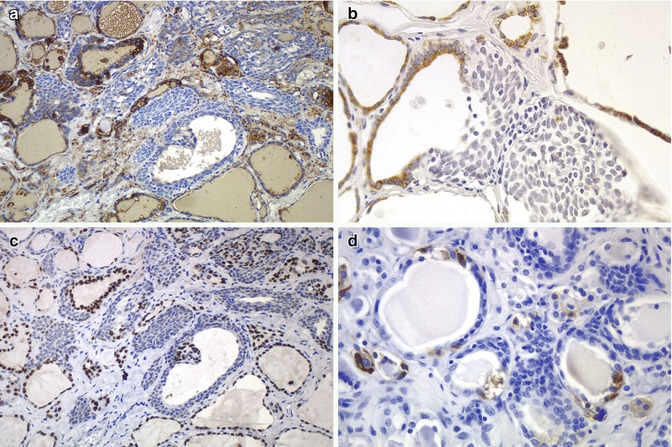

Fig. 6.6
Immunohistochemical features of solid cell nests (SCNs). Main cells of human SCNs have strong cytoplasmic positivity for cytokeratin 19 (a) and BCL-2 protein (b). They are also immunoreactive for p63 (c) and carcinoembryonic antigen (CEA) (d)

Fig. 6.7
Immunohistochemical features of solid cell nests (SCNs). SCNs show negativity for thyroglobulin by immunohistochemistry (a) and in situ hybridization (mRNA) (b). TTF-1 is also negative in main cells of SCNs (c). Positivity for calcitonin is limited to the C cells usually associated to the SCNs (d)
SCNs are thought to be remnants of the fifth branchial arch (UB) [1, 2]. The UB origin of SCNs is supported by similarities with the UB of mammals, its relation with C cells (Fig. 6.8), production of mucins [7], association with branchial-like cysts (Figs. 6.9 and 6.10), presence in some piriform sinus fistula as well as the occurrence of SCNs along with thymic, parathyroid, adipose, fibrous, cartilaginous and salivary gland-type tissues (Fig. 6.11).
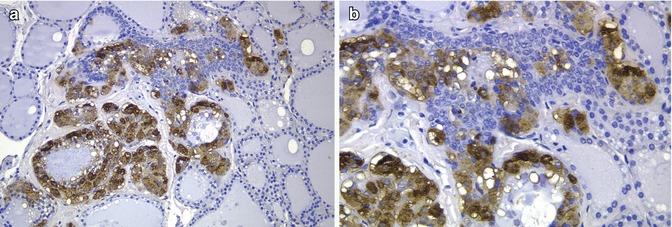
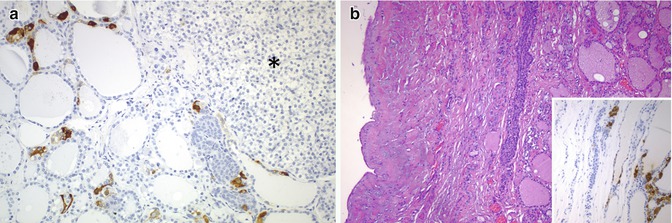
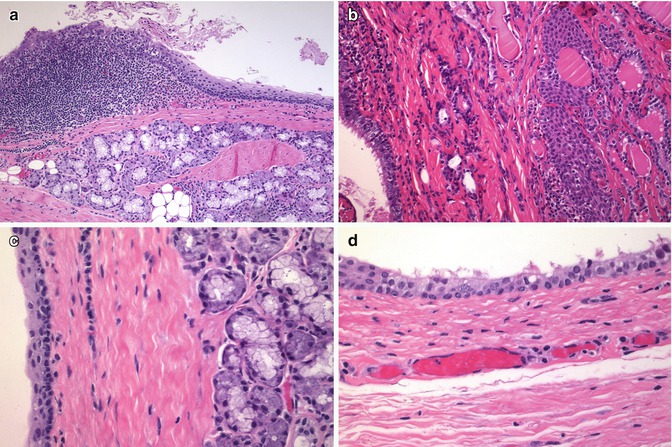
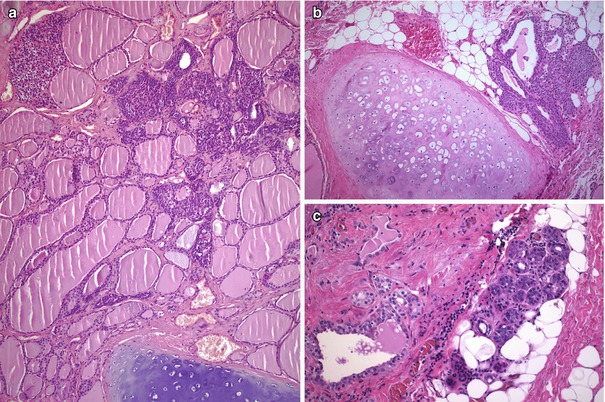

Fig. 6.8
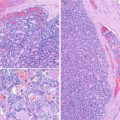
Solid cell nests in a patient with multiple endocrine neoplasia type 2A (MEN 2A). In this case associated to MEN 2A, calcitonin staining showed a very high number of C cells associated with SCNs (a, b). Primary C-cell hyperplasia is associated with heritable forms of medullary thyroid carcinoma. In fact, there is evidence to support the view that primary C-cell hyperplasia in patients with MEN2 syndromes and in some sporadic microcarcinomas represents a thyroid intraepithelial neoplasia of C cells

Fig. 6.9
C cells in solid cell nests (SCNs) and branchial-like cysts. Numerous C cells stained for calcitonin are associated with SCNs adjacent to the ectopic (intrathyroidal) parathyroid tissue (asterisk) (a). SCNs accompanied by numerous calcitonin-positive C cells (inset) are seen on the wall of this case of intrathyroidal branchial-type cyst (b)

Fig. 6.10
Intrathyroidal branchial cleft cyst. Lymphoid aggregates (a), solid cell nests (b) and salivary gland-type tissue (c) were found in the wall of a branchial cleft cyst occurring in the right thyroid lobe of a children. The wall of the cyst was lined by stratified squamous (a, c) and ciliated respiratory (b, d) epithelium

Fig. 6.11
Solid cell nests (SCNs) with intrathyroidal parathyroid, cartilage and salivary gland tissue. The presence of tissues of the branchial apparatus such as parathyroid tissue (a), islands of cartilage (a, b) and salivary gland-type tissue (c) around SCNs supports the assumption of a branchial origin for SCNs
In contrast to foci of squamous metaplasia which are generally associated with inflammatory or reparative phenomena, SCNs usually appear in normal thyroid tissue accompanied by C cells (Fig. 6.12). Squamoid cell nests of Hashimoto’s thyroiditis [8] share some nuclear features with PTC, but they are neither neoplastic structures nor true SCNs. Such squamoid cell nests are usually smaller than SCNs and display floret-like features, surrounded by a collection of lymphocytes, having weak TTF-1 positivity and negativity for HBME-1 and galectin-3 and lacking associated C cells [8] (Fig. 6.13). The squamoid cell nests of Hashimoto’s thyroiditis seem to represent ductal metaplasia, a type of phylogenic regression to an exocrine structure, secondary to chronic injury [9]. Rare cases of Hashimoto’s thyroiditis massive squamous metaplasia of follicular cells [10] and multiple branchial cleft-like cysts (cystic variant of Hashimoto’s thyroiditis) [11] may occur (Fig. 6.14). True isolated intrathyroid branchial cysts have also been reported (Fig. 6.10). In comparison to branchial derived cysts, true keratinizing cysts similar to epidermoid type inclusion cyst are extremely rare in the thyroid (Fig. 6.15).
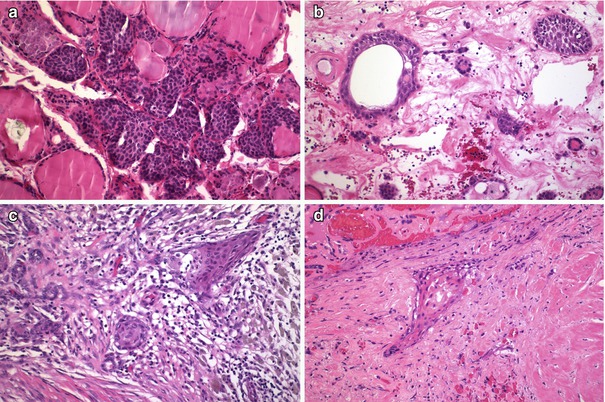
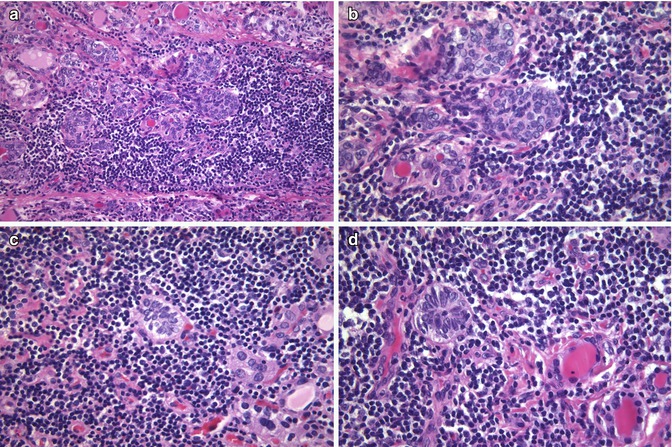
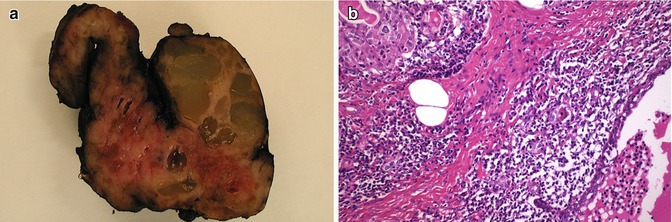
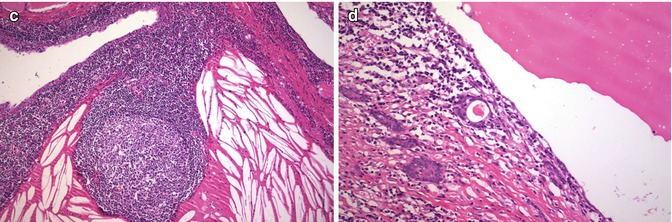
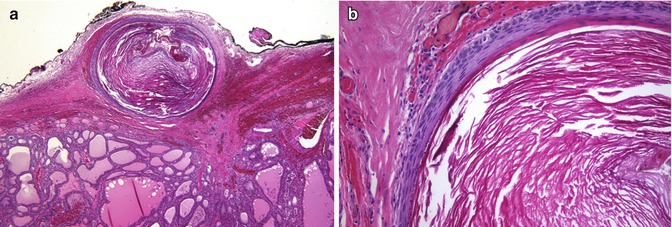

Fig. 6.12
Solid cell nests (SCNs) and squamous metaplasia. SCNs usually appear in normal thyroid tissue, sometimes accompanied by scattered lymphocytes (a). In contrast, nests of squamous metaplasia of follicular cells usually appears in a stroma with oedema, fibrin, dense mixed inflammatory infiltrate with abundant histiocytes and variable fibrosis (b–d)

Fig. 6.13
Ductal metaplasia in chronic lymphocytic thyroiditis . Compared to branchial-derived SCNs, the cell nests that occur in thyroiditis are usually smaller squamoid nests of cells with “floret-like” features, surrounded by a collection of lymphocytes and lacking associated C cells (a–d). These squamoid nests of Hashimoto’s thyroiditis are considered to represent ductal metaplasia, secondary to chronic injury. Ductal metaplasia cells are strongly positive for p63 , stain weakly for TTF-1 and are negative for thyroglobulin, HBME-1 and calcitonin . These immunophenotypic features can be used to distinguish ductal metaplasia from papillary thyroid microcarcinomas (strongly positive for thyroglobulin, TTF-1 and HBME-1 and variably positive for p63) associated with Hashimoto’s thyroiditis


Fig. 6.14
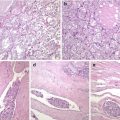
Cystic variant of chronic lymphocytic (Hashimoto) thyroiditis . Rare cases of chronic lymphocytic thyroiditis show numerous cystic formations some of which are evident in macroscopic examination (a). In this case of a 50-year-old woman, microscopic examination revealed several branchial cleft-like cysts containing dense eosinophilic material with cholesterin clefts and lymphocytes. The cysts were lined by stratified epithelium of variable thickness. In the stroma, areas of diffuse lymphoplasmacytic infiltration, germinal centres and oncocytic metaplasia alternated with marked fibrosis, follicular atrophy and squamous metaplasia (b–d)

Fig. 6.15
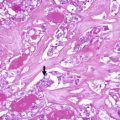
Keratinous (epidermoid type) cyst. Keratinous cyst located on the surface of the gland surrounded by fibrous tissue of the capsule (a). This cyst is similar to an epidermoid type inclusion cyst; it is lined by cornified epithelium, has a distinct granular layer and contains lamellated keratin (b). Since the patient had a previous fine needle aspiration biopsy (FNAB), it is tempting to propose that the cyst has resulted from traumatic inclusion of epidermis secondary to the FNAB
It is possible that mixed follicles [12] may represent a phenomenon of asymmetric division (Fig. 6.16). We found positivity for CD133 in SCNs and in some follicles in the vicinity of a case of SCNs (unpublished observations) (Fig. 6.16). OCT4 protein expression, however, was only detected in a few cases of SCNs [13]; further research is therefore required to confirm their stem cell role.
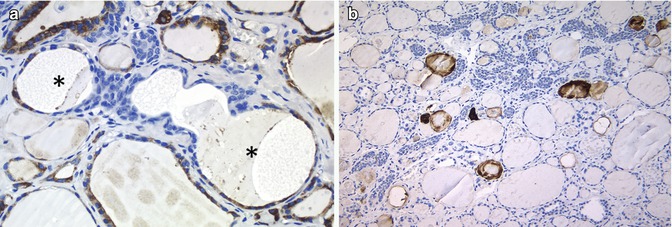

Fig. 6.16
Stem cell features in solid cell nests (SCNs). Main cells of the SCNs apparently harbour the minimal properties of a stem cell phenotype (telomerase activity and differentiation to one or more than one type of specialized cells) and may thus represent a pool of stem cells of the adult thyroid. In SCNs, the follicles (asterisk) lined by main cells and follicular epithelium positive for thyroperoxidase (mixed follicles) could represent the so-called asymmetric division of stem cells (a). CD133, thought to be a stem cell marker, is also positive in SCNs (b)
One hyperplastic (giant) SCN case has been described [14]. Although no BRAF gene mutations are found in normal SCNs, BRAF V600E mutation has been reported in a case of diffuse and bilateral SCN hyperplasia with a contiguous papillary microcarcinoma also having the same BRAF V600E mutation [15]. In this case, two PTCs and the SCNs associated with the first micro-PTC showed the same BRAF mutation, while the follicular adenoma, the normal follicular cells and three other samples of SCNs showed no mutation. These findings support the assumption that SCN hyperplasia may be a precursor lesion of PTC [15]. In a similar way, our group reported in the centre of a follicular variant of PTC a second neoplastic lesion featuring a SCN-like appearance sharing a Q61R mutation in the N-RAS gene with the follicular variant of PTC [16]; this finding supports also a histogenetic relationship between these two lesions (see Chap. 2). Other studies have also supported a histogenetic link between SCNs and PTC, as well as with sclerosing mucoepidermoid carcinoma [4, 8]. A case of papillary thyroid microcarcinoma coexisting in intimate relationship with SCNs is illustrated in Fig. 6.17.
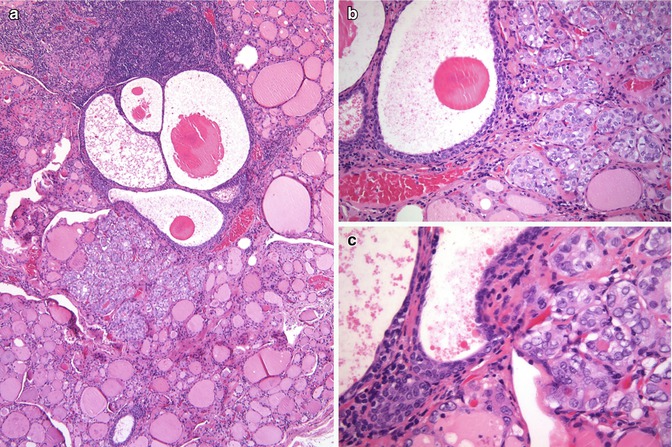

Fig. 6.17
Solid cell nests (SCNs) and papillary thyroid carcinoma. This figure illustrates a case of papillary thyroid microcarcinoma coexisting in intimate relationship with SCNs (a–c). In comparison to SCNs, the papillary microcarcinoma shows a pattern of microfollicular growth and larger nuclei with clearer chromatin
An additional relationship between SCNs and carcinoma showing thymus-like differentiation of the thyroid (CASTLE) has recently been reported [17]. Regardless of whether or not the main cells of SCNs serve as neoplastic stem cells, the broad spectrum of differentiation of SCNs may explain conceptually the existence of primary thyroid tumours with “aberrant” phenotypes.
A case of thyroid-type SCNs associated with struma ovarii has been reported supporting a histogenetic link between the main cells of SCNs and thyroid tissue [18]. Cystic tumour of the atrioventricular node of the heart [19] and the so-called complex choristoma of the gyrus rectus in central nervous system [20] are two intriguing lesions, morphologically and immunophenotypically equivalent to SCNs of the thyroid.
Spindle Epithelial Tumour with Thymus-Like Differentiation (SETTLE)
SETTLE is a rare, malignant tumour of the thyroid characterized by a lobulated architecture and biphasic cellular composition featuring spindly epithelial cells that merge into glandular structures [5, 21–23] (Figs. 6.18, 6.19, 6.20 and 6.21). It is thought that this tumour is derived from the branchial pouches or thymic remnants and shows primitive thymic differentiation [22]. It occurs predominantly in euthyroid children and young adults. SETTLE are usually limited by a continuous capsule with fibrous septa although they may also have an infiltrative growth pattern. Vascular invasion is rarely observed. The spindle cells are usually bland, and the epithelial (or epithelioid) cells may form tubules, small papillae, trabeculae or squamoid nests (Figs. 6.18, 6.19 and 6.20). Occasional cases comprise almost exclusively spindly cells or glandular structures, the so-called monophasic variant of SETTLE. The epithelial cells are cuboidal to columnar and are sometimes mucinous or ciliated (Figs. 6.20 and 6.21). The spindle cell component expresses low and high molecular weight cytokeratins, CK5, p63 , vimentin and, often, CD99 , while the epithelioid component usually lacks vimentin and CD99 expression (Fig. 6.19). Thyroglobulin, calcitonin , TTF-1, carcinoembryonic antigen , CD5 and CD34 are usually negative in both components. The differential diagnosis of SETTLE includes spindle cell variant of follicular adenoma (Fig. 3.9), spindle cell variant of PTC (Fig. 2.10) and spindle cell variant of MTC, ectopic thymoma , solitary fibrous tumour (Figs. 6.25 and 6.26) and synovial sarcoma . The distinction between SETTLE and synovial sarcoma may be achieved only after the exclusion of the t(X,18) rearrangement typical of synovial sarcoma.
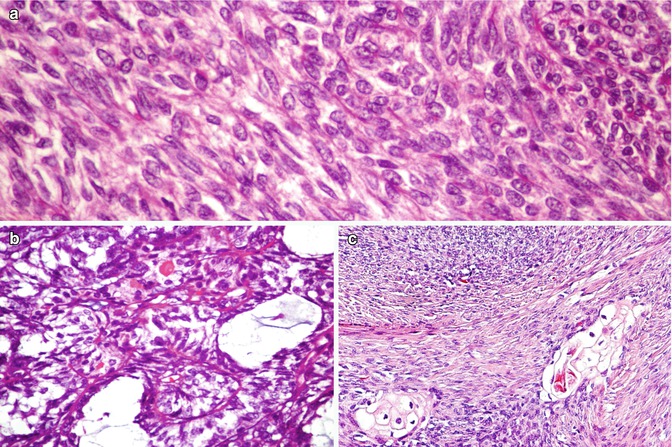
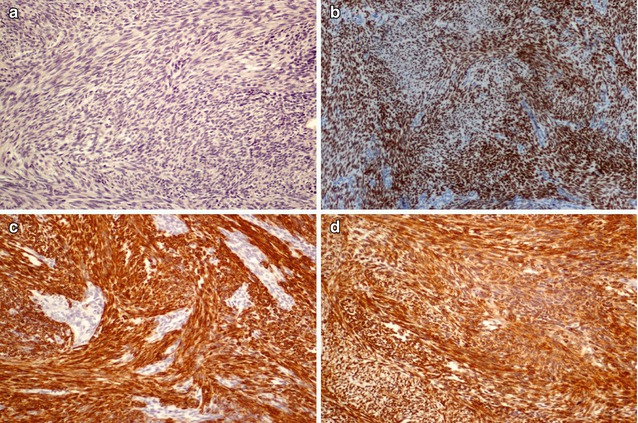
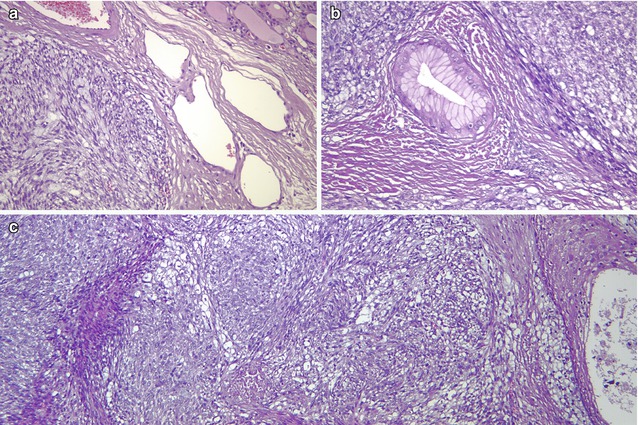

Fig. 6.18
Spindle epithelial tumour with thymus-like differentiation (SETTLE) . Biphasic pattern of SETTLE including a bland spindle cell component (a) and an epithelial component that may disclose glandular structures with mucin cysts (b) and squamoid nests (c)

Fig. 6.19
Immunohistochemical features of SETTLE . The bland spindle cell component of SETTLE does not express thyroglobulin (a) and expresses p63 (b), pankeratins (clone AE1/AE3) (c) and Bcl-2 (d)

Fig. 6.20




SETTLE . This neoplasm is characterized by a lobulated architecture (a) and biphasic cellular composition featuring spindly epithelial cells that merge into glandular (b) and squamous (c) epithelium. The hypercellular and monotonous quality of the spindle cell component may lead to a misdiagnosis of synovial sarcoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree