Other Mesenchymal Lesions
Most pathologic entities in the breast arise from the epithelium. However, the components of the mammary stroma may also give rise to various lesions which, in general, are similar in appearance to comparable lesions seen at other sites.
Many benign and malignant mesenchymal lesions that occur elsewhere in the body have been described in the breast. Only those more frequent or important from the point of view of differential diagnosis are described in this chapter.
BENIGN MESENCHYMAL LESIONS
Lipoma
Lipomas of the breast present clinically and radiographically as circumscribed soft tissue masses. Given that adipose tissue is a normal component of breast stroma and can be abundant, a diagnosis of lipoma of the breast is difficult in the absence of a low-power appearance of a nodule of mature adipose tissue surrounded by a capsule. In fact, we rarely make this diagnosis in our clinical practice.
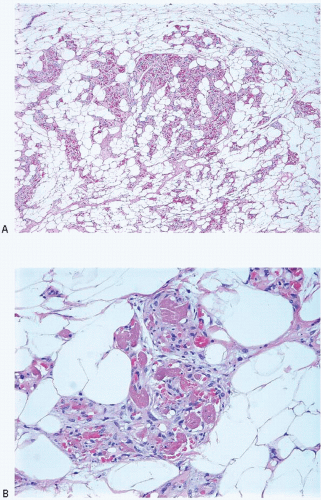
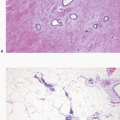
Angiolipomas also occur in the breast. As for angiolipomas elsewhere, the distinguishing feature is the presence within a lipomatous tumor of small blood vessels containing fibrin thrombi within a delicate fibrous stroma (Fig. 13.1, e-Fig. 13.1). Cellular angiolipomas are characterized by a cellular spindle cell proliferation in which the vessels may be collapsed and difficult to appreciate and in which the adipose tissue component may be sparse (Fig. 13.2).1,2 In addition, these lesions can exhibit cytologic atypia and rare mitoses.2 The appearance of blood vessels in adipose tissue as seen in angiolipomas may raise the concern for a vascular lesion, including angiosarcoma, particularly in core-needle biopsy samples. Often excision is required to render a definitive diagnosis, particularly for cellular angiolipoma.2 Other lipoma variants, such as fibrolipoma and spindle cell lipoma, have also been described in the breast.3
It should be noted that lipomatous tumors submitted to the pathologist as specimens from the breast are, in fact, frequently from the subcutaneous tissue overlying the breast rather than from the mammary parenchyma per se.
Some authors have described adenolipomas in which normal mammary glandular structures are incorporated within the substance of an apparent lipoma. These lesions are probably better categorized as hamartomas (see Chapter 6).
Granular Cell Tumor
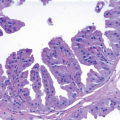
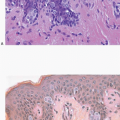
Granular cell tumors are uncommonly found in the breast but, when present, can simulate carcinoma on clinical examination, imaging studies, and gross pathologic examination.4, 5 and 6 These tumors occur more commonly in African-American than in Caucasian women6,7 and typically appear between puberty and menopause, implicating hormonal factors in their development. Granular cell tumors of the breast most commonly occur in the upper, inner quadrant in contrast to carcinomas, which occur most frequently in the upper, outer quadrant. Patients present with a palpable mass that may be associated with skin retraction or fixation to skeletal muscles of the chest wall.
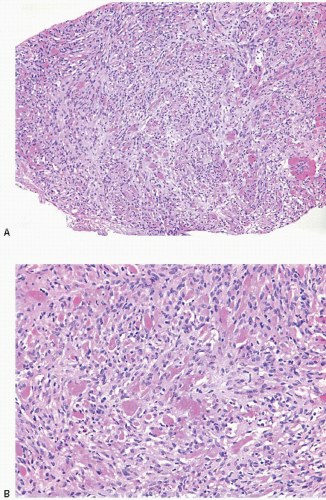
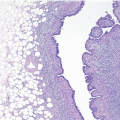
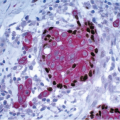
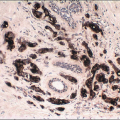
Gross examination reveals a gray-white to tan firm tumor that may be gritty when cut; these features further give the impression of carcinoma. Microscopically, these lesions are identical to granular cell tumors in other sites, consisting of a poorly circumscribed, infiltrative proliferation of cells in which the most characteristic feature is prominent granularity of the cytoplasm (Fig. 13.3, e-Fig. 13.2). Upon electron microscopic examination, these granules correspond to secondary lysosomes. The nuclei are small and uniform and lack the features of malignancy. The differential diagnosis of granular cell tumor includes fat necrosis and other processes with an accumulation of histiocytes (such as duct ectasia), as well as invasive carcinomas. Granular cell tumors are positive for S100 protein (Fig. 13.3), negative for cytokeratin, and negative for estrogen receptor and progesterone receptor expression.
Granular cell tumors are almost invariably benign and are adequately treated by local excision.6 Rare cases of malignant granular cell tumors have been reported in both the breast and extramammary sites. Although initially considered to be myogenic in nature (hence, their earlier designation as granular cell myoblastomas), ultrastructural and immunohistochemical evidence has proven that these are of neurogenic origin.4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree