Fig. 19.1
Incidence of invasive head and neck cancer in AYAs (age 15–39), 2000–2011, SEER18, by anatomical site
19.1.1 Incidence
Figure 19.2 shows the incidence of head and neck (oropharyngeal; oral cavity and pharynx: lip, tongue, salivary gland, floor of the mouth, gum and other mouth, nasopharynx, tonsil, oropharynx, hypopharynx, other oral cavity, and pharynx) cancer during 2000–2011 in the United States as a function of age and sex. The incidence of head/neck cancer had a distinct sigmoid relationship with age. From age 10 to 40, the increase was exponential (Fig. 19.2, inset). Above the age of 30, males had a greater incidence of head/neck cancer; by age 40–50, the incidence was twice that in females. Before age 30, the incidence in males and females was the same.
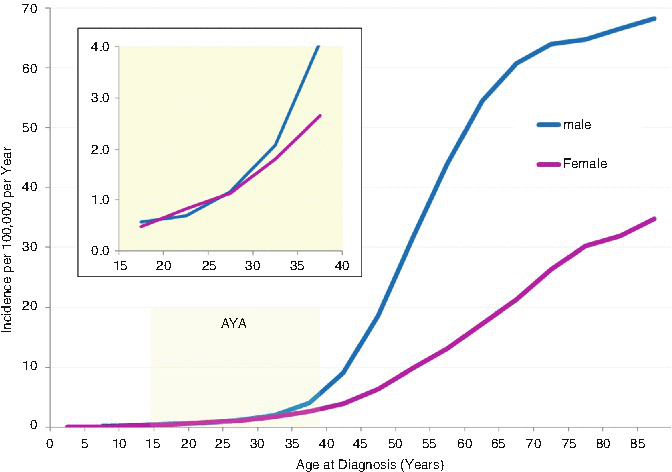

Fig. 19.2
Incidence of invasive head and neck cancer, 2000–2011, SEER18, by stage and age. The inset depicts the AYA age range
Figure 19.3 depicts the incidence in the United States as a function of extent of disease at diagnosis. In AYAs, the most common stage at diagnosis was localized (inset), whereas in older persons, it was a regional disease. Less than 10 % of AYAs presented with distant metastases (Fig. 19.3, inset). In situ histology accounted for only 2 % of head/neck cancer in AYAs (inset). In AYAs, Asians/Pacific Islanders had the greatest incidence of head/neck cancer and native North Americans the least. This is in contrast to persons of older age in whom non-Hispanic whites and blacks distinctly had the highest incidence. In AYAs, males had 56 % of the malignant head/neck cancers, Asians/Pacific Islanders had 32 %, non-Hispanic white had 24 %, and blacks had 21 % of the cancers.
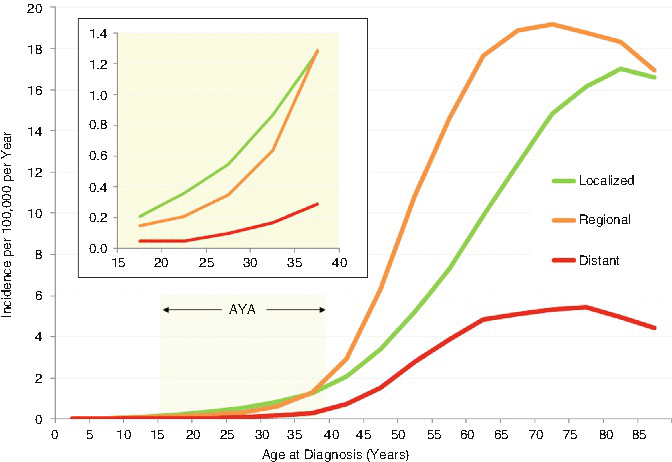

Fig. 19.3
Incidence of invasive head and neck cancer, 2000–2011, SEER18, by stage and age. The inset depicts the AYA age range
During 2000–2012, Asians/Pacific Islanders in the United States had the highest incidence of head/neck cancer in AYAs, in contrast to the non-Hispanic whites and blacks in older adults (Fig. 19.4). Hispanics had the lowest incidence at all ages (Fig. 19.4).
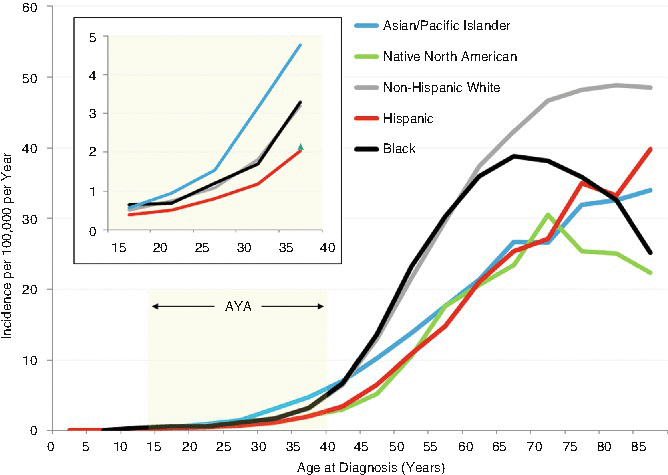

Fig. 19.4
Incidence of invasive head and neck cancer, 2000–2011, SEER18, by stage and age. The inset depicts the AYA age range
The proportion of head/neck cancer that is Epstein–Barr virus associated (EBV+) and human papillomavirus associated (HPV+) (Table 19.1) is greater in AYAs than in any other age group [1, 2]. Nearly half of head/neck cancers in AYAs are HPV+.
Table 19.1
Proportion of head/neck carcinoma that is HPV+, 2005, by age at diagnosis
Age at diagnosis (years) | ||||
|---|---|---|---|---|
<45 | 45–54 | 55–64 | 65+ | |
Incidence HPV–a | 0.5 | 5.8 | 8.5 | 12.0 |
Incidence HPV+a | 0.4 | 9.0 | 20.0 | 30.0 |
% HPV+ | 44 % | 39 % | 30 % | 29 % |
19.1.2 Incidence Trends
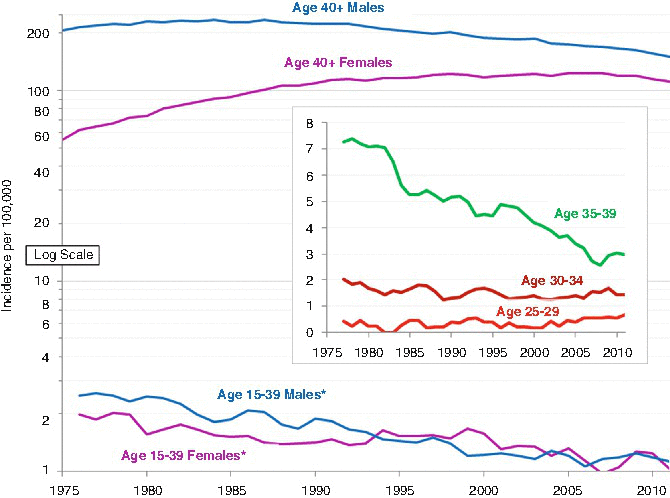
Whereas the incidence of head/neck cancer in both males and females older than 40 decreased steadily during the 1980s and 1990s, attributed to the decline in smoking in the United States, the incidence of head/neck cancer in AYAs from 1975 to 2011 was relatively constant in males; it increased steadily in females, however (Fig. 19.5). The lack of continued decrease in older adults since 2000 is disappointing and may indicate a failure of anti-smoking campaigns since then. All of the age subgroups in AYAs have had a constant incidence of head/neck cancer since 1975.
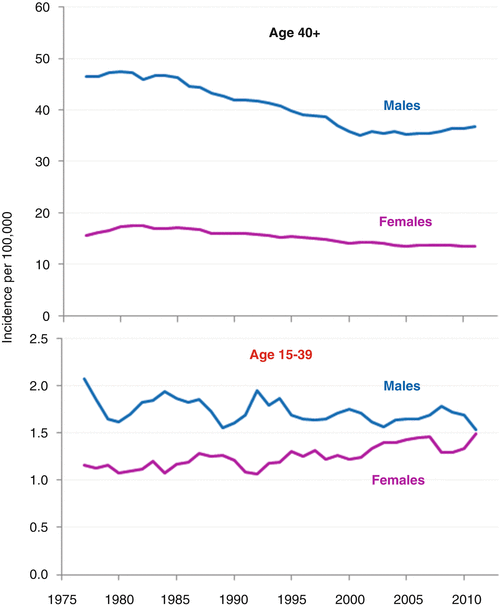

Fig. 19.5
Annual incidence of invasive head and neck cancer in AYAs (age 15–39) and older adults (age 40+), 2000–2011, SEER18, by sex 3-year moving averages
In the United States, the incidence of cancer in the oral cavity and pharynx was reduced successfully with public health programs that diminished the prevalence of tobacco use. In 2007, the prevalence of smoking dropped below an age-adjusted age rate of 20 % from as high as 57 % for men in 1955 and 34 % for women in 1965 [3, 4]. Recently, however, this progress in reducing the incidence of oropharyngeal cancer has declined in some population subgroups as the tobacco-related cancers have been replaced by those related to the human papillomavirus (HPV) [5, 6]. HPV, the causal factor in nearly all carcinomas of the uterine cervix, is also known to have a role in the pathogenesis of squamous cell carcinomas of the oral cavity and pharynx [7–15]. In vitro, HPV serotypes 16 and 18 are capable of transforming epithelial cells derived from both the genital and upper respiratory tracts [16], and HPV has been demonstrated to alter p53 and Rb human oncogenes [17, 18]. The higher incidence of oropharyngeal cancer in females than males is consistent with orogenital sexual activity that puts females at higher risk of HPV transmission.
The increase in oropharyngeal cancer is apparent in females as young as 15 to 19 years of age [19]. The oldest 5-year age group demonstrating the acceleration was the 30- to 34-year-olds [19]. The recent acceleration in incidence among females is most obvious from age 15 to 34 [19]. The increase in females was due primarily to squamous, mucoepidermoid, and acinar carcinomas and not adenocarcinoma or other epithelial and glandular carcinomas of the head and neck [19]. The incidence trends were statistically significant for the squamous and acinar morphologies [19]. The anatomic sites within the oral cavity and pharynx that were most affected by the increase in females were the salivary glands and tongue, followed by the nasopharynx and tonsils [19]. The oropharynx and other sites classified in the ICD-O-3 designation did not demonstrate an increase in the age group. When the last decade is compared with prior decades, nearly half of the increase occurred in the salivary glands and more than a third in the tongue [19].
The decreasing incidence of oropharyngeal cancers since the 1980s in both men and women [20] was reversed in females less than 40 years of age in the early 1990s and resulted in an accelerating increase at least until the most recent year of available data. This pattern coincides with the national increase in orogenital sexual intercourse in young females and earlier age of sexual intercourse, in part because oral sex has had increased acceptability among adolescents and young adults and in some it is an alternative to vaginal intercourse. In an outpatient setting, the risk for developing HPV infection in 18- to 23-year-olds was found to increase with the number of lifetime oral sex partners (p < 0.007) [20].
Specific sexual behaviors have been associated more strongly with the risk of an HPV+ tumor, including a history of performing oral sex and oral–anal contact [21–24]. In a hospital-based population, patients who reported having had one to five oral sex partners were 3.8 times more likely to have an HPV-related oropharyngeal cancer, and if six or more partners were reported, the increase was 8.6-fold (95 % confidence interval of 2.2–34.0 times) [11].
That the most common anatomic sites within the oral cavity and pharynx that were affected by the increase in incidence in females were the salivary glands and tongue is also consistent with a transmission of a virus by direct topical contact via the genital–oral route. Other sites that contributed to the increase are the nasopharynx and tonsils. Sites that have not had an increase in females include the floor of the mouth, oropharynx, and hypopharynx, which are not involved as directly with this route of transmission. On the other hand, the majority of HPV+ tumors in males with oropharyngeal cancer have been reported previously to occur mainly in the lingual and palatine tonsils of the oropharynx [8, 9, 17, 25–30]. The difference with the results of the study reported here is probably due to the fact that oropharyngeal predilection may be limited to males [21] and this study demonstrates an overall increase only in females. The incidence trends observed in this report may implicate the more anterior compartment of the oropharyngeal carcinoma in the pathogenesis of HPV-related, genital–oral-induced human neoplasia. Most of the increase in females was due to squamous carcinoma and, in the salivary glands, mucoepidermoid and acinar carcinomas. The predilection for squamous epithelium is consistent with what has been reported previously as the tropism for HPV [10, 12, 13, 18].
The dramatic decrease in the incidence of oropharyngeal cancer in 25- to 39-year-old males is likely due to the reduction in tobacco use and reversal of the HIV/AIDS epidemic. The decrease in older persons, both male and female, is likely primarily due to decreased tobacco use, which in males includes chewing tobacco. The increasing rate of oropharyngeal carcinoma in young females found in this study does not appear to have been reported previously. That a second order polynomial regression had the best fit with the available data is consistent with a reversal in incidence trend. The shape of the curve – of a decrease followed by an increase – implies an etiologic shift for which decreasing tobacco exposure being replaced by an increasing sexually transmitted disease is a reasonable explanation. The youngest females demonstrating an increase in oropharyngeal carcinoma, 10–19-year-olds, had the least evidence for a trend reversal increase, with 10–14-year-olds having had a linear trend. Since it is unlikely that tobacco exposure results in oropharyngeal cancer this early in life, the trends are consistent with a new oncogenic etiology in the age group such as HPV. That such a young age group is affected by HPV-induced cancer is consistent with the decreasing age of onset of sexual activity and with a more than twofold greater risk of oropharyngeal carcinoma when the age of first sexual intercourse is before 18 in comparison to 18 or older [11].
All of the racial/ethnic groups had evidence for the trend reversal in young females. That Asians had the highest incidence of oropharyngeal carcinoma is consistent with known epidemiologic patterns that in older persons have been attributed to a high prevalence of cigarette smoking and tobacco chewing [31]. Non-Hispanic whites appear to have had the greatest increase since the 1990s, and Hispanics/Latinos have had the lowest rates, racial/ethnic differences that may be attributable to orosexual behaviors.
HPV 16-positive oropharyngeal cancer has been associated with an increasing number of oral sex partners [11] and with increasing marijuana use and not with tobacco smoking and alcohol drinking. Reciprocally, HPV 16-negative oropharyngeal cancer has not been associated with sexual behavior or marijuana use, but has been associated with tobacco and alcohol use [32]. The HPV+ patient also appears to be distinct from the HPV− patient with regard to alcohol and tobacco exposure history. HPV+ oropharyngeal cancer is more likely than HPV− oropharyngeal cancer to occur in the nonsmoker and nondrinker [13, 17, 21, 27, 33, 34]. In a study restricted to patients with oropharyngeal cancers, nonsmokers were approximately 15-fold more likely to have a diagnosis of HPV+ oropharyngeal cancer than smokers [35]. Similarly, several studies have reported an inverse association between HPV status and alcohol use [17, 27, 33, 36, 37].
19.1.3 Survival
During 2000–2011 in the United States, AYAs had a better overall 5-year oropharyngeal cancer survival than older and, to the extent evaluable, younger patients (Fig. 19.6). They also had a better stage-per-stage survival (Fig. 19.6 inset), with 5-year cancer-specific survivals of >90 % for stage I and 40–60 % for stage IV. At all ages from 10 to 80 years, males had a worse 5-year head/neck-cancer-specific survival rate than females. At all ages above 10, the 5-year head/neck-cancer-specific survival was strongly and directly dependent on stage at diagnosis. Among AYAs, the 5-year survival of those with distant disease at diagnosis was 50 %, whereas those with localized disease had a >90 % 5-year survival.
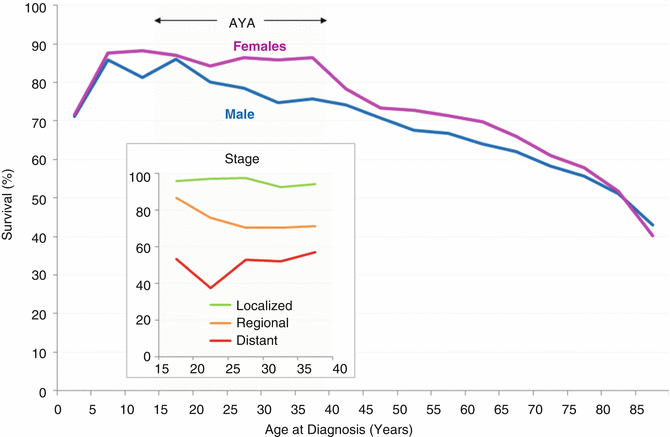

Fig. 19.6
5-year cancer-specific survival of patients with invasive head and neck cancer, 1975–2011, SEER18, by sex, stage (inset), and age; only age intervals with >10 patients are shown
The 5-year head/neck-cancer-specific survival of AYAs with head/neck cancer was independent of race/ethnicity with the exception that non-Hispanic white AYAs had a relatively better survival rate (Fig. 19.7). In older adults, blacks had the worst survival rate.
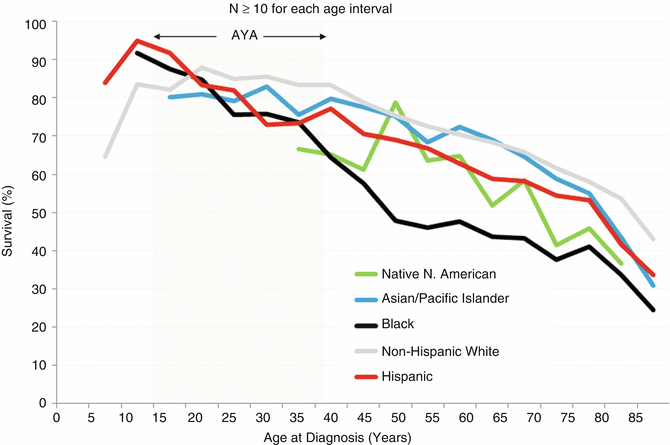

Fig. 19.7
5-year cancer-specific survival of patients with invasive head and neck cancer, 1975–2011, SEER18, by sex, stage (inset), and age; only age intervals with >10 patients are shown
The 5-year head/neck-cancer-specific survival of AYAs was distinctly worse for those who presented with hypopharyngeal primaries (Fig. 19.8). It was best for those with primaries of the salivary glands, lip, gum, and tonsil.
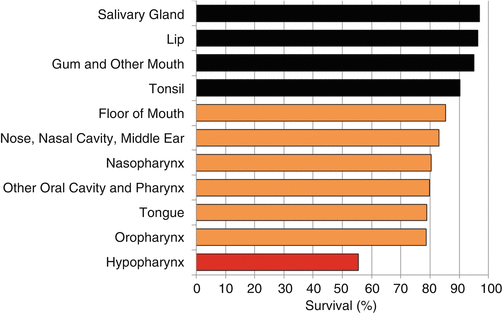

Fig. 19.8
5-year cancer-specific survival of patients with invasive head and neck cancer, 1975–2011, SEER18, age 15–39, by anatomical location
19.1.4 Survival Trends
During 1976–2011 in the United States, AYA males have had less improvement in the 5-year head/neck-cancer-specific survival rate than older adults, but AYA women have had virtually no improvement in the 5-year head/neck-cancer-specific survival rate. Black and Asian/Pacific Islander AYAs with head/neck cancer have had an improvement in their 5-year cancer-specific survival rate, whereas white AYAs have not and Hispanic AYAs appear to have suffered a worse survival with each 8-year interval since 1976.
There is evidence that patients with HPV-related oropharyngeal carcinoma have better long-term survival than those with HPV-unrelated head and neck squamous cell carcinoma (e.g., 5-year overall survival rate of >80 % versus ∼40 % for patients with stage III-IV tumors) [38]. This is especially true with tumors of the oropharynx [32, 37, 39–42]. This may be due in part to the fact that patients with HPV-related tumors are younger, with fewer comorbidities, and are less often alcoholics and smokers [17, 27, 36, 39, 40]. It may be also that the underlying biology of the HPV-transformed cell and its Rb- and p53-based [17, 18, 43] mutations render the carcinoma that results more prognostically favorable or because of other age-based biological distinctions summarized recently [42], resulting in a different type of cancer in adolescents and young adults than in older patients. In the analysis reported here, the increase in incidence in adolescent and young adult females with oropharyngeal cancer was not associated with an improvement in survival, especially in comparison with older females and with males who have had a 17–24 % increase in 5-year survival during the past 25 years (Fig. 19.2, lower panel). If the increase in oropharyngeal cancer among adolescent and young adult females is due to HPV-mediated tumors, the higher reported survival rates may be limited to those receiving treatment at academic cancer centers that are studying and treating head and neck cancer, rather than in the general population as represented by the SEER registries. It could be also that the patients reported to have a better survival are older than the age most affected by the increasing incidence. Thus far, nearly all of the HPV-associated cases reported to have a better survival were over 40 years of age when diagnosed [30, 37, 40, 41].
The relative lack of survival progress in young adult females with oropharyngeal carcinoma is consistent, however, with the relative lack of progress in the SEER database for all cancer in the age group relative to younger and older patients [44] The inability to apply progress in older (and younger) patients to the adolescent and young adult age group, which has been ascribed to lack of health insurance, delayed diagnosis, low clinical trial participation, psychosocial challenges, and multiple factors unique to these patients [45], may just as well apply to oropharyngeal cancer as it does to other malignancies. Given the roles of tobacco, alcohol, and HPV in the oropharyngeal cancer population, it may well be that many of these factors are more problematic.
19.1.5 Mortality
Figure 19.9 summarizes the head/neck cancer death rates in AYAs during 2000–2011 in the United States according to sex, race, and anatomical site of the primary tumor. Nearly twice as many AYA males died of head/neck cancer than AYA females. Among AYAs with head/neck cancer, native North Americans and Asians/Pacific Islanders had the greatest death rate, followed by blacks and, with the lowest rate, whites. By far, most AYAs who died of head/neck cancer had primary cancer in the tongue and nasopharynx. Cancers of the oropharynx, hypopharynx, lip, and floor of the mouth were uncommon causes of death in AYAs.
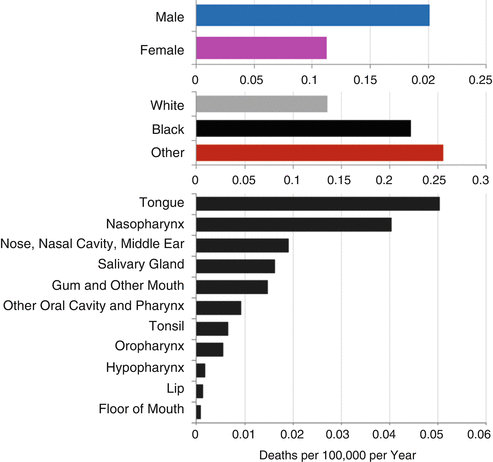

Fig. 19.9
5-year death rate of AYAs (age 15–39) with invasive head and neck cancer, 1975–2011, SEER18, by sex, race/ethnicity, and anatomical site
19.1.6 Risk Factors
Tobacco use and alcohol consumption are the main risk factors associated with head/neck SCC in older persons, due to their cytotoxic and mutagenic effects on the exposed epithelia of the upper aerodigestive tract. In AYAs, Epstein–Barr virus (EBV) and high-risk human papillomaviruses (HPVs), both encoding viral oncoproteins able to interfere with cell cycle control, have been recognized as the etiological agents of nasopharynx carcinoma and oropharyngeal carcinoma, respectively [46]. EBV and HPV transmission occurs predominantly via oral–oral (saliva and upper respiratory secretions) and orogenital routes, respectively.
19.1.7 Biology
The World Health Organization classification has three basic types of head/neck carcinomas: squamous cell carcinoma (SCC), non-keratinizing carcinoma, and undifferentiated carcinoma. The non-squamous types predominate in AYAs, whereas SCC occurs primarily in older adults. The molecular heterogeneity of head/neck SCC includes methylation profiles, microRNA expression, and mutated genes that may represent new targets for cancer-tailored therapies [46]. Most nasopharyngeal and some oropharyngeal carcinomas in AYAs are EBV+, whereas oropharyngeal carcinomas are more likely to be HPV+. [The other carcinoma that is likely to be EBV+ is gastric carcinoma, reviewed later in this chapter.] The biology of HPV+ oropharyngeal cancer is distinct: p53 degradation, retinoblastoma pathway inactivation, and p16 upregulation [47].
19.1.8 Treatment
Because the majority of AYAs present with early-stage disease, head/neck cancer in most AYAs patients is usually treated with surgery and postoperative radiotherapy or chemoradiotherapy. The minority of AYAs with advanced-stage head/neck cancer receive postoperative cisplatin-based chemotherapy and, increasingly, molecularly targeted therapy such as anti-epidermal growth factor receptor (EGFR) therapy. Most patients with HPV+ oropharyngeal carcinoma or EBV+ nasopharyngeal carcinoma present with cystic nodal metastases with a small primary tumor and respond well to all treatment modalities including primary surgery and chemoradiotherapy [48]. As of 2016, cetuximab was the only FDA-approved anti-epidermal growth factor receptor therapy for the treatment of head/neck SCC. A number of monoclonal antibodies targeting AKT, mTOR, and PI3K pathways and therapeutic vaccines against HPV 16 and EBV proteins are under evaluation. Furthermore, virus-associated oropharyngeal cancers may benefit from new developed immunotherapies targeting HPV E6 and E7 oncoproteins [49]. The higher survival rate also renders AYAs more likely to experience chronic therapy-induced morbidity [38]. Current research is evaluating de-escalation of treatment and follow-up evaluation to reduce long-term treatment-associated morbidities [48, 50]. In patients with EBV+ nasopharyngeal cancer, plasma levels of EBV DNA are both prognostic and helpful in guiding postoperative chemotherapy [51, 52].
The more favorable survival of AYAs with head/neck cancer is due in part to the more favorable subtypes of HPV+ and EBV+ carcinomas in the AYA population. This biologic difference also provides preventive opportunities.
19.1.9 Prevention
The recent increase in oropharyngeal cancer among females escalates the need for HPV immunization, for which two vaccines are available in the United States, one a quadrivalent vaccine licensed in 2006 and the other a bivalent vaccine approved in 2009 [53]. Both protect against the HPV serotype 16 that accounts for the majority of HPV-related oropharyngeal cancers [54]. The bivalent is recommended for use in females aged 10 through 25 years and the quadrivalent for females aged 11 or 12 years and catch-up vaccination for females aged 13 through 26 years [54]. Immunizing males should also help by reducing the transmission from males to females and to males who also engage in oral sex and for persons in whom the route of contagion may be oral-oral rather than orogenital [55–57]. Both vaccines also have a high efficacy against HPV 16- and 18-related cervical precancer lesions. HPV 4 also has high efficacy against HPV 6- and HPV 11-related genital warts and HPV 16- and 18-related vaginal and vulvar precancer lesions [53].
The societal challenge is to reduce the burden of HPV-related disease, estimated to be 25,000 cases of cancer annually in the United States [58], by vaccination against certain disease-inducing strains of the virus, combined with the community’s interest in limiting the transmission of infectious diseases while promoting health on the one hand and social mores on the other [59].
19.2 Lung Cancer in AYAs in the United States
19.2.1 Incidence
During 2000–2011 in the United States, AYA males and females had a similar incidence of lung cancer, beginning at 15 years of age, whereas in adults older than 50 year, males had a greater incidence of lung cancer (Fig. 19.10). In AYAs the increase was exponentially dependent on age. For 2000–2011, an average of nearly 3,000 AYAs was diagnosed annually with lung cancer (Table 19.2). It is not a rare cancer in the older AYA age range.
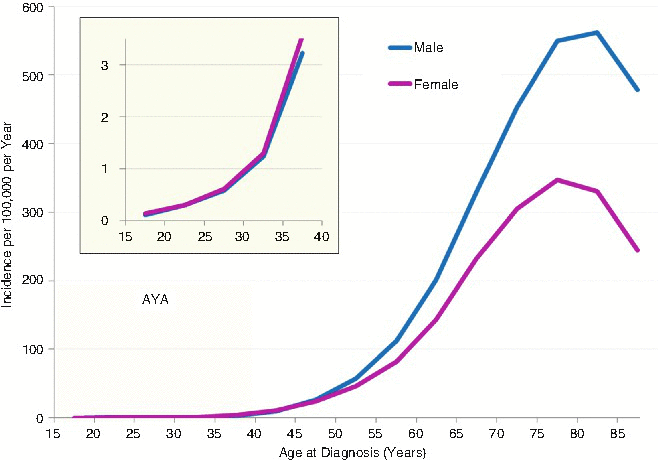

Fig. 19.10
Incidence of cancer of the lung and bronchus, 2004–2011, SEER18, by age and sex. The inset depicts the AYA age range
Table 19.2
Average annual number of AYAs diagnosed to have gastric cancer in the United States, 2000–2011
Age (years) | |||||
|---|---|---|---|---|---|
15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 16–39 |
65 | 144 | 321 | 716 | 1,597 | 2,844 |
Figure 19.11 shows the stage of lung cancer at diagnosis as a function of patient age in the United States, according to the AJCC 6th edition staging system since 2004. At all ages above 20, the most common stage at diagnosis of lung cancer was stage IV. The most common stage at diagnosis of lung cancer at all ages was localized disease. 60 % of AYAs diagnosed with lung cancer had stage IV disease.
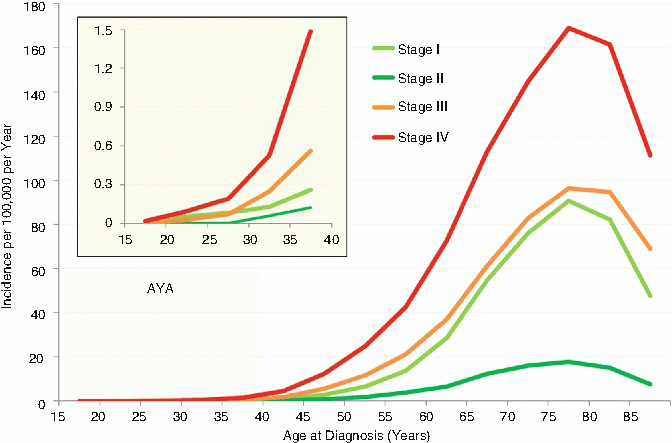

Fig. 19.11
Incidence of cancer of the lung and bronchus, 2000–2011, SEER18, by age and stage. The inset depicts the AYA age range
Figure 19.12 depicts the incidence of lung cancer in the United States as a function of race/ethnicity and age. The incidence among AYAs, Asians, non-Hispanic whites, and blacks had the highest incidence of lung cancer, and Hispanics and native North Americans had the lowest incidence. Among older persons, blacks had the highest incidence, followed by non-Hispanic whites. Among AYAs as a group, females had a slightly higher incidence of lung cancer. Among AYAs, blacks, non-Hispanic whites, and Asians/Pacific Islanders had approximately twice the rate of lung cancer incidence of Hispanics and native North Americans.
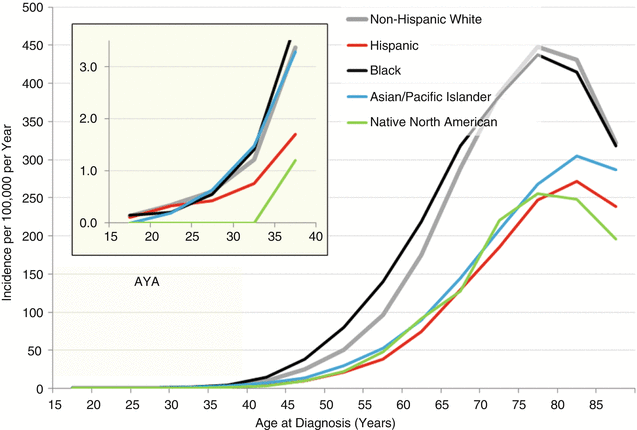

Fig. 19.12
Incidence of cancer of the lung and bronchus, 2000–2011, SEER18, by age and stage. The inset depicts the AYA age range
Figure 19.13 shows the type of lung carcinoma in AYAs as function of age. Carcinoid and neuroendocrine carcinomas were far more prevalent in AYAs than in any older age group. Most 15- to 24-year-olds were diagnosed with either carcinoid or neuroendocrine carcinoma. AYAs were also more likely to be diagnosed with bronchoalveolar carcinoma than at any other age. Among the non-small cell lung cancers, adenocarcinoma and non-squamous tumors are more common in AYAs than in older patients [60, 61]. Non-small cell lung cancer of the anaplastic lymphoma kinase rearrangement is more common in AYAs than in older patients [62].
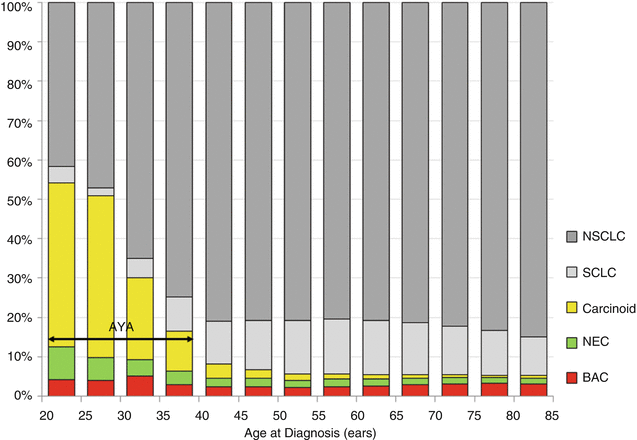

Fig. 19.13
Incidence of cancer of the lung and bronchus, 2000–2011, SEER18, by age and histologic type. NSLC non-small cell lung carcinoma, SCLC small cell lung carcinoma, NEC neuroendocrine carcinoma, BAC bronchoalveolar carcinoma
19.2.2 Incidence Trends
Figure 19.14 portrays the change in lung cancer incidence from 1976 to 2011 in the United States in AYAs and older patients. In older males, the incidence of lung cancer declined steadily from 1990 to 2011, whereas in older females, the incidence increased steadily, especially during the 1970s and 1980s and more slowly during the 1990s and early 2000s. For the first time during the past half century, the incidence in older females showed evidence for a decrease, since 2007. In both AYA males and females, however, the incidence steadily declined since 1975, especially in males. In females AYAs, the decline in incidence attenuated during the 1990s such that both male and females AYAs had the same incidence since the mid-1990s. Among AYAs, the decline in incidence of lung cancer was limited to the 35- to 39-year-old age group. In 25- to 34-year-olds, the incidence was constant since 1980 (Fig. 19.14 inset).