Several new oral drugs that selectively and directly inhibit factor Xa seem promising alternatives to existing antithrombotic drugs. These drugs have a convenient route of administration, can be given in fixed doses, and do not require coagulation monitoring. Favorable results of clinical trials support their potential to change current practice. Translation into daily clinical practice may take some time; clinical studies over the next months and years will reveal the impact of rivaroxaban and other compounds in development. The aim of this review is to provide an overview of the more advanced oral, direct factor Xa inhibitors and to briefly describe the results of the completed studies.
The approach to the development of new anticoagulants as alternatives to heparins and vitamin K antagonists (VKAs) has been guided by the requirement for convenient administration with predictable pharmacokinetics (PK), pharmacodynamics (PD), and a wide therapeutic window that permits fixed dosing without requiring coagulation monitoring. Research has focused in particular on targeting thrombin and factor Xa (FXa), which are common to the intrinsic and extrinsic coagulation pathways. Thus, thrombin inhibitors act to prevent fibrin formation as well as inhibit thrombin-mediated activation of factors V, VIII, XI, and XIII and platelets. Inhibitors of FXa act at an earlier stage in the cascade. They inhibit free and prothrombinase-bound FXa and are also able to inhibit clot-associated FXa, thus preventing clot-associated FXa from activating prothrombin.
After the development of parenteral direct thrombin inhibitors, such as lepirudin or bivalirudin, ximelagatran was the first direct orally available direct thrombin inhibitor. Ximelagatran was withdrawn in 2006 after concerns about liver toxicity. The oral direct thrombin inhibitor, dabigatran etexilate, has recently been approved for use in the European Union and Canada for thromboprophylaxis after total hip replacement surgery (THR) and total knee replacement surgery (TKR) and is currently in an advanced stage of development for other indications. Fondaparinux, a parenteral indirect FXa inhibitor, was the first drug to prove the efficacy of selective FXa inhibition in the prevention and treatment of thromboembolic disease. Several oral FXa inhibitors are now under investigation for the prevention and treatment of thromboembolic disorders, of which rivaroxaban and apixaban are in the most advanced stage of development.
The aim of this review is to provide an overview of the more advanced oral, direct FXa inhibitors and to briefly describe the results of the completed studies.
Rivaroxaban
Rivaroxaban (BAY 59-7939) is a potent and selective oral FXa inhibitor with a particular chemical structure in its active-site binding region that plays a role in the oral absorption of the drug, with a relatively high bioavailability (nearly 80%). Rivaroxaban has a rapid onset of action with predictable, dose proportional PK and PD. Plasma levels of the drug peak after 3 to 4 hours, with a mean half-life ranging from 5 to 9 hours in young individuals and from 11 to 13 hours in the elderly. The main route of excretion is renal, but the drug is also excreted via the fecal/biliary route. Phase I studies demonstrated that gender and body weight did not have a clinically relevant influence on the PK and PD of rivaroxaban in healthy subjects, suggesting that this drug could be administered at a fixed dose in any patient. Coadministration with food intake only minimally increases peak plasma concentrations of rivaroxaban, and only a few potential interactions with other drugs have been shown. Rivaroxaban is metabolized in the liver by cythocrome and noncythocrome mechanisms and more than 30% of the drug is excreted in the feces as unchanged drug. Because this phenomenon is, at least in part, mediated by P-glycoprotein, drugs that concomitantly act as strong inhibitors of cytochrome CYP3A4 and P-glycoprotein, such as azole antifungal and HIV-protease inhibitors, significantly interfere with the metabolism of rivaroxaban and increase its plasma concentration.
Rivaroxaban for the Prevention of Venous Thromboembolism
In phase II, dose finding studies, rivaroxaban was compared with the low molecular weight heparin (LMWH), enoxaparin, for the prevention of venous thromboembolism (VTE) in patients undergoing THR and TKR; in two studies, rivaroxaban was administered twice a day, with a total daily dose ranging from 5 to 60 mg, and in a subsequent study, rivaroxaban was given once a day, with a dosage ranging from 5 to 40 mg. Overall, rivaroxaban was well tolerated by the patients, and daily doses between 5 and 20 mg showed efficacy and tolerability that were similar to those of enoxaparin; major bleeding rates increased only with higher doses of rivaroxaban. On the basis of these results, the 10-mg dose administered once daily was selected for subsequent phase III studies. Based on the results of phase I studies and of phase II studies, no dose adjustments according to age, body weight, or gender were considered necessary. Rivaroxaban showed the potential to offer clinicians the option of an oral compound that can be administered at a fixed, unmonitored, once-daily dose. The Regulation of Coagulation in Major Orthopedic Surgery Reducing the Risk of DVT and PE (RECORD) program comprised four phase III studies in which rivaroxaban was compared with enoxaparin for the prevention of VTE in more than 12,500 patients undergoing THR and TKR.
In RECORD 2, patients undergoing THR were randomized to oral rivaroxaban (10 mg once daily for 35 [±4] days; extended prophylaxis) or to subcutaneous enoxaparin (40 mg once daily for 12 [±2] days; short-term prophylaxis); in RECORD 1, rivaroxaban and enoxaparin, at the same dosages as in RECORD 2, were administered for 35 (±4) days (extended prophylaxis) in patients undergoing THR. In RECORD 3 and RECORD 4, rivaroxaban and enoxaparin were administered to patients undergoing TKR for 12 (±2) days, as was recommended by the American College of Chest Physicians guidelines at the time when the studies were planned (2004 guidelines). RECORD 3 was conducted primarily in Europe and patients, according to the European standard of practice, received enoxaparin (40 mg once daily) beginning the evening before surgery. Conversely, RECORD 4 was conducted primarily in North America and enoxaparin (30 mg twice-daily) was started 12 to 24 hours after surgery according to the standard of practice. In all four studies, rivaroxaban (10 mg once daily) was started 6 to 8 hours after surgery.
Efficacy and safety endpoints were identical in all trials. The primary efficacy endpoint was represented by the occurrence of total VTEs, defined as any deep vein thrombosis (DVT), nonfatal pulmonary embolism (PE), and all-cause mortality. Secondary efficacy outcomes included major VTEs (ie, proximal DVT, nonfatal PE, or death related to VTE) and symptomatic VTE. The main safety endpoint was major bleeding occurring after the first blinded dose and until up to 2 days after the last dose of study medication. Major bleeding was defined as bleeding that was fatal or involved a critical organ or required reoperation or as clinically overt bleeding outside the surgical site that was associated with a decrease in the hemoglobin level of greater than or equal to 2 g/dL or requiring an infusion of greater than or equal to 2 units of blood. Also assessed were nonmajor bleeding, including hemorrhagic wound complications (excessive wound hematoma or bleeding at the surgical site), other adverse events, including liver toxicity and cardiovascular events, and death.
In RECORD 2, the incidence of the primary efficacy outcome was significantly reduced from 9.3% in the group of patients receiving short-term enoxaparin to 2.0% in the group of patients receiving long-term rivaroxaban (relative risk reduction [RRR] 79%, P <.0001). The incidence of major and symptomatic VTEs were also significantly reduced from 5.1% to 0.6% (RRR 88%, P <.0001), and 1.2% to 0.2% (RRR 80%, P = .004), respectively.
In RECORD 1, total VTEs were reduced from 3.7% in the group of patients receiving enoxaparin to 1.1% in the group of patients receiving rivaroxaban (RRR 70%, P <.001), and the incidence of major VTEs was reduced from 2.0% to 0.2%, respectively (RRR 88%, P <.001). Symptomatic VTEs occurred in 0.5% in the enoxaparin group and in 0.3% of patients in the rivaroxaban group.
In RECORD 3, all primary and secondary efficacy endpoints were reduced with the use of rivaroxaban. Total VTEs were reduced from 18.9% in the group of patients receiving enoxaparin to 9.6% in the group of patients receiving rivaroxaban (RRR 49%, P <.0001), major VTEs from 2.6% to 1.0% (RRR 62%, P = .010), and symptomatic VTEs from 2.0% to 0.7%, respectively (RRR 66%, P = .005).
Finally, RECORD 4 demonstrated that rivaroxaban was more effective than enoxaparin administered at a dosage of 30 mg once daily for the prevention of total VTEs (rivaroxaban 6.9% vs enoxaparin 10.1%, RRR 31%, P = .012). The rates of major VTEs and symptomatic VTEs with rivaroxaban and enoxaparin were 1.2% versus 2.0% and 0.7% versus 1.2%, respectively.
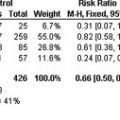
In all RECORD studies rivaroxaban showed a similar rate of major bleeding events and clinically relevant nonmajor bleeding events, when compared with enoxaparin. This was also supported by the absence of laboratory signs of compromised liver function attributable to rivaroxaban. The results of the RECORD studies are summarized in Table 1 .
| Study | Patient Group (n) | % Primary Efficacy Endpoint (Composite of Any DVT, Nonfatal PE, and All-Cause Mortality) | % Relative Risk Reduction ( P value) | % Primary Safety Endpoint: Major Bleeding (Number) e |
|---|---|---|---|---|
| RECORD1 a, Extended prophylaxis with oral rivaroxaban versus extended sc enoxaparin | THA (4541) | |||
| Rivaroxaban (10 mg od) | 1.1 (18/1595) | 70 ( P <.001) | 0.3 (6/2209) | |
| Enoxaparin (40 mg od) | 3.7 (58/1558) | 0.1 (2/2224) | ||
| RECORD2 b, Extended prophylaxis with oral rivaroxaban versus short-term sc enoxaparin | THA (2509) | |||
| Rivaroxaban (10 mg od) | 2.0 (17/864) | 79 ( P <.001) | <0.1 (1/1228) | |
| Enoxaparin (40 mg od) | 9.3 (81/869) | <0.1 (1/1229) | ||
| RECORD3 c, Thromboprophylaxis with oral rivaroxaban versus sc enoxaparin | TKA (2531) | |||
| Rivaroxaban (10 mg od) | 9.6 (79/824) | 49 ( P <.001) | 0.6 (7/1220) | |
| Enoxaparin (40 mg od) | 18.9 (166/878) | 0,5 (6/1239) | ||
| RECORD4 d, Thromboprophylaxis with oral rivaroxaban versus sc enoxaparin | TKA (3148) | |||
| Rivaroxaban (10 mg od) | 6.9 (67/965) | 31 ( P = .012) | 20.7 (10/1526) | |
| Enoxaparin (30 mg bid) | 10.1 (97/959) | 0.3 (4/1508) |
a Oral, once-daily rivaroxaban (10 mg), started 6–8 hours after surgery, versus sc once-daily enoxparin (40 mg), started the evening before surgery. Both regimens continued for 36 (±6) days.
b Oral, once-daily rivaroxaban (10 mg), started 6–8 hours after surgery, for 35 (±4) days, versus sc once-daily enoxaparin (40 mg), started the evening before surgery, continued for 12 (±2) days, followed by placebo.
c Oral, once-daily rivaroxaban (10 mg), started 6–8 hours after surgery for 12 (±2) days, versus sc once-daily enoxaparin (40 mg), started the evening before surgery, continued for 12 (±2) days, followed by placebo.
d Oral, once-daily rivaroxaban (10 mg), started 6–8 hours after surgery for 12 (±2) days, versus sc twice-daily enoxaparin (30 mg), started the evening before surgery, continued for 12 (±2) days, followed by placebo.
e Major bleeding was defined as bleeding that was fatal, involved a critical organ, or required reoperation or clinically overt bleeding outside the surgical site that was associated with a decrease in the hemoglobin level of ≥2 g/d or requiring an infusion of ≥2 units of blood.
A clinical trial evaluating rivaroxaban for the prevention of VTE in high-risk medical patients, the Multicenter, Randomized, Parallel Group Efficacy and Safety Study for the Prevention of Venous Thromboembolism in Hospitalized Medically Ill Patients Comparing Rivaroxaban with Enoxaparin (MAGELLAN) study, is currently ongoing. In MAGELLAN, a double-blind, double-dummy study, patients are randomized to receive rivaroxaban administered at a dosage of 10 mg once daily for 35 (±4) days or enoxaparin 40 mg once daily for 10 (±4) days.
Rivaroxaban for the Treatment of VTE
In addition to thromboprophylaxis, rivaroxaban is also being evaluated for the treatment of VTE. Two phase II, dose finding studies compared rivaroxaban administered at total daily doses ranging from 20 to 60 mg with standard therapy with LMWH followed by oral VKAs. Based on the positive results of these studies, three phase III clinical trials aimed to assess the acute phase and the long term treatment of DVT and PE have been initiated. Rivaroxaban dosages of 15 mg twice daily for 3 weeks followed by 20 mg once daily are used in the ongoing EINSTEIN-DVT and EINSTEIN-PE studies. These studies enroll patients with objectively confirmed, symptomatic DVT or PE who are randomized to treatment with rivaroxaban alone or with LMWH and VKAs for a total period of 3 to 12 months. The EINSTEIN-Extension study treats patients with rivaroxaban (20 mg once daily) or placebo after 6 to 12 months of treatment with VKAs or rivaroxaban. This study is completed, and the results have been presented at the American Society of Hematology meeting in December 2009. The primary efficacy outcome was the recurrence of symptomatic VTE and the principal safety outcome was the occurrence of major bleeding. A total of 1197 patients were randomized between February 2007 and May 2009. The intention-to-treat population consisted of 602 rivaroxaban and 594 placebo patients. The mean duration of study treatment was 190 days in both groups. During the treatment period, symptomatic recurrent VTE events occurred in 42 (7.1%) patients treated with placebo and in 8 (1.3%) patients treated with rivaroxaban (hazard ratio, 0.18; 95% CI, 0.09–0.39). After stopping the study medication, 6 (1.0%) symptomatic recurrent VTE events occurred in both groups during the 1-month observational period of follow-up. No major bleeding events were documented in the group of patients treated with placebo; 4 (0.7%) major bleeding events occurred in the rivaroxaban group ( P = .106). None of these bleeding events was fatal or occurred in a critical site. Clinically relevant nonmajor bleeding occurred in 7 (1.2%) and in 32 (5.4%) patients randomized to placebo and rivaroxaban, respectively. Two (0.3%) patients in the placebo group and 1 (0.2%) patient in the rivaroxaban group died. No patients had documented liver toxicity defined as an alanine aminotransferase rise above 3 times the upper limit of normal combined with a total bilirubin above 2 times the upper limit of normal. Thus, the fixed dose of rivaroxaban (20 mg once daily) administered for the long-term secondary prevention of VTE resulted in a significant reduction in the recurrence of VTE as compared with no treatment, and, most of all, active therapy with rivaroxaban was not associated with an increased incidence of major bleeding events.
Rivaroxaban for the Prevention and Treatment of Arterial Thrombosis
The 20-mg once-daily dose of rivaroxaban was also chosen in the Randomized, Double-Blind Study Comparing Once Daily Oral Rivaroxaban with Adjusted-Dose Oral Warfarin for the Prevention of Stroke in Subjects with Non-Valvular Atrial Fibrillation (ROCKET AF) study, a phase III study on the long-term prevention of stroke in patients with atrial fibrillation ( www.clinicaltrials.gov ; NCT00403767). In the ROCKET AF study, patients with high-risk, nonvalvular atrial fibrillation are randomized to treatment with warfarin, administered to achieve an international normalized ratio (INR) range between 2.0 and 3.0, or to treatment with rivaroxaban. In this study, a lower dose of rivaroxaban (ie, 15 mg once daily) is administered to patients with a creatinine clearance of between 30 and 49 mL per minute. The enrollment phase of the ROCKET AF study is now completed, and the results should be available by the end of 2010.
Finally, rivaroxaban was also tested in patients with acute coronary syndrome (ACS) in a phase II study. The Rivaroxaban in Combination with Aspirin Alone or with Aspirin and Thienopyridine in Subjects with Acute Coronary Syndromes (ATLAS ACS-TIMI 46) trial is a randomized, double-blind, placebo-controlled dose escalation study created to assess the safety and efficacy of rivaroxaban in patients with ACS. In this study patients receiving aspirin (stratum 1) or aspirin plus thienopyridine (stratum 2), after the acute coronary event, were randomized to receive placebo or rivaroxaban, administered at a total daily dose (range, 5–20 mg) given once or twice daily. The primary safety endpoint was clinically relevant bleeding; the primary efficacy endpoint was the time to the first episode of death, myocardial infarction, stroke, or severe recurrent ischemia requiring revascularization. Clinically relevant bleeding occurred in a dose-dependent manner: compared with placebo, rivaroxaban was associated with a significant increase in bleeding rates, with hazard ratios (HRs) ranging from 2.21 (CI, 1.25–3.91) for the 5-mg dose to 5.06 (3.45–7.42) for the 20-mg dose. The increase in bleeding across doses was apparent in both strata, but the absolute rates of clinically relevant bleeding were lower in stratum 1 than in stratum 2. Compared with placebo, treatment with rivaroxaban resulted in a hazard ratio (HR) of 3.96 (CI, 1.40–11.23) for clinically relevant bleeding in stratum 1 and of 3.66 (CI, 2.54–5.27) in stratum 2. Across the entire population, rates of the primary efficacy endpoint were 5.5% in the group receiving rivaroxaban and 7.0% in the group receiving placebo (HR 0.79; CI, 0.60–1.05), with this risk reduction being statistically significant in stratum 1 (HR 0.53; CI, 0.33–0.84), but not in stratum 2 (HR 0.99; CI, 0.9–1.42). On the basis of these observations, a larger, phase III clinical trial was then planned with the aim to analyze the efficacy of rivaroxaban at the doses of 2.5 mg and 5 mg administered twice daily in ACS patients ( www.clinicaltrials.gov ; NCT00809965).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree