Therapy-related risk factors
Chemotherapy and biotherapy agents
Antimetabolites
Cytarabine
5-fluorouracil
Methotrexate
Antitumor antibiotics
Actinomycin D
Amsacrine
Mithramycin
Mitomycin
Alkylating agents
Busulfan
Cyclophosphamide
Mechlorethamine
Procarbazine
Thiotepa
Anthracyclines
Daunorubicin
Doxorubicin
Epirubicin
Taxanes
Paclitaxel
Docetaxel
mTOR inhibitors
Hematopoietic stem cell transplant
Allogeneic > autologous transplant
Radiation therapy
Incidence and severity related to site and fractionation
Combination therapy
Chemotherapy + radiation therapy
Patient-related risk factors
Age
Children have an increased risk of mucositis due to increased cell turnover
Genetic factors
Genes associated with chemotherapy metabolism may play an important role in increased development of mucositis
Oral health and hygiene
A comorbidity of dental or oral infection may complicate mucositis
Nutritional status
Poor nutritional status may affect increased breakdown and delayed healing
Smoking
Affects circulation and may delay healing
Previous experience
History of mucositis can predict future mucositis
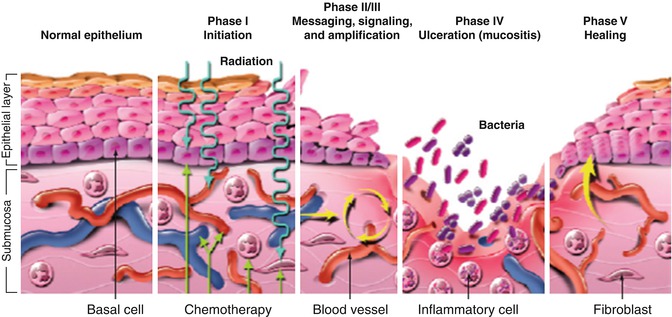
11.2.2 Pathophysiology of Oral Mucositis
Mucositis has been described as a complex physiologic process that can be examined in five stages: initiation, primary damage response, signal amplification, ulceration, and healing (Fig. 11.1).
11.2.2.1 Initiation
The initiation stage occurs immediately following the administration of radiation or chemotherapy. In this stage, direct damage to both DNA and non-DNA occurs. This initial insult to tissues does not represent the extensive injury that characterizes the clinical presentation of mucositis. Chemotherapy agents and radiation therapy generate reactive oxygen species (ROS) that cause damage to connective tissue, DNA, and cell membranes, stimulate macrophages, and trigger a cascade of critical biologic mechanisms and molecular pathways including p53, nuclear factor kappa-B (NF-κB), and the ceramide pathway (Sonis 2004, 2011). At this point, the tissues have a normal clinical appearance.
11.2.2.2 Primary Damage Response
The primary damage response phase is an extremely active phase associated with significant inter- and intracellular signaling in connective tissue, endothelium, and the submucosa (Sonis 2011). Activated transcription factors mediate both gene expression and the synthesis and release of biologically active mediators that impair the viability of the basal epithelium (Sonis 2007). NF-κB is thought to be the most significant transcription factor in the development of mucositis. It is responsible for regulating the expression of approximately 200 genes, many of which play a role in the pathogenesis of mucositis; specifically, it is a regulator of proinflammatory cytokines including tumor necrosis factor α (TNF-α), IL-6 and IL-1β. These proinflammatory cytokines secondarily cause damage that ultimately results in apoptosis of epithelial basal cells (Tresiter and Sonis 2007).
11.2.2.3 Signal Amplification
During signal amplification cytokine mediators generated during the primary damage response phase provide positive feedback resulting in a cascade of damaging mediators. These feedback loops reinitiate the damage response pathways. An example of this is NF-κB stimulation by TNF-α to continue its downstream activity (Sonis 2004, 2011). These feedback loops magnify the response of the initial injury by amplifying and potentiating the original biologic signals, increasing tissue injury and prolonging damage by continuing to provide signals for days after the original chemotherapeutic or radiation insult (Sonis 2007).
11.2.2.4 Ulceration
Ulceration is the phase with the most clinical significance. Ulceration develops as a consequence of the direct and indirect mechanisms noted in the previous phases that cause damage and apoptotic changes to mucosal epithelium (Sonis 2009). Mucosal integrity is lost, resulting in painful ulcers. The ulcers are covered by a pseudomembrane and are a desirable environment for secondary bacterial colonization. Neutropenic patients are at greater risk of sepsis due to the Gram-positive and Gram-negative organisms that thrive in the pseudomembrane (Sonis 2007).
11.2.2.5 Healing
In the absence of infection, healing is usually complete within 2–3 weeks. The healing phase is the least understood of the phases of mucositis but is thought to be controlled by signaling from the submucosal mesenchyme to the epithelium. Signaling controls migration, differentiation and proliferation of new tissue across the base of the ulcer (Treister and Sonis 2007).
11.2.3 Clinical Course of Oral Mucositis
On initial presentation of OM, the oral mucosa first shows erythema which then progresses to erosion and ultimately ulceration. Ulcerations are typically covered by a white fibrinous pseudomembrane. Lesions will heal approximately 2–4 weeks after the last dose of the offending chemotherapy or radiation therapy. In the case of immunosuppressed patients, resolution of OM usually coincides with granulocyte recovery (Lalla et al. 2008). Chemotherapy-induced OM is limited to nonkeratinized surfaces such as the lateral and ventral tongue, buccal mucosa, and soft palate (Lalla et al. 2008). Radiation-induced OM occurs in the radiation field with nonkeratinized tissue affected more often. Patients receiving increasing cumulative doses beyond 30 Gy will be more severely affected (Sonis 2007; Lalla et al. 2008). The clinical course of mucositis can be complicated by infection, particularly in the immunocompromised patient.
11.3 Assessment and Measurement of Oral Mucositis
Historically, mucositis measurement and assessment in pediatrics have relied on tools developed for adults (Tomlinson et al. 2009). Reliable oral assessment is essential to facilitate management strategies or implement clinical trials in mucositis prevention and treatment (Tomlinson et al. 2007, 2009). The scales currently utilized to assess pediatric OM do not address practical issues such as mechanisms for optimal visualization of the oral cavity and approaching an uncooperative child. There are also limitations to reporting subjective and functional domains in the child (Tomlinson et al. 2007).
Simple instruments such as the World Health Organization (WHO) grading system and the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events are scales that range from 0 (with no symptoms) to 4 or 5 (worst symptoms possible) (Fig. 11.2). Generally these scales are based on the ability to eat and drink combined with objective signs of mucositis. These instruments are utilized by pediatric oncology groups as part of toxicity criteria measurement. They are seen to be the most relevant scales for clinical management as a global score is achieved readily (Sonis et al. 2004). However, these simple scales may underreport mucositis if effective analgesia is administered to the patient (Sonis 2009).
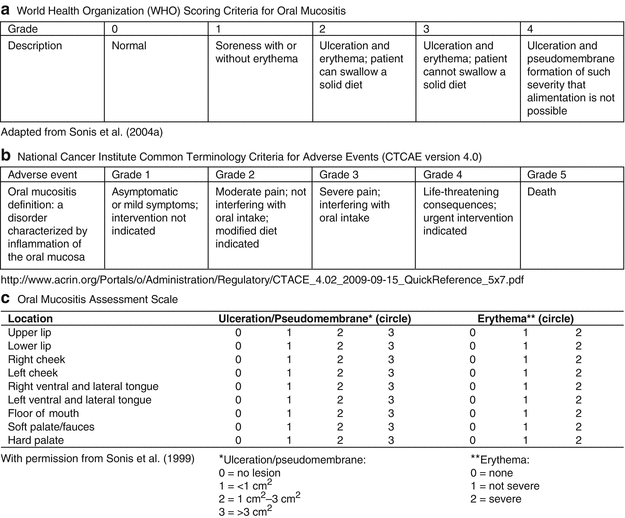
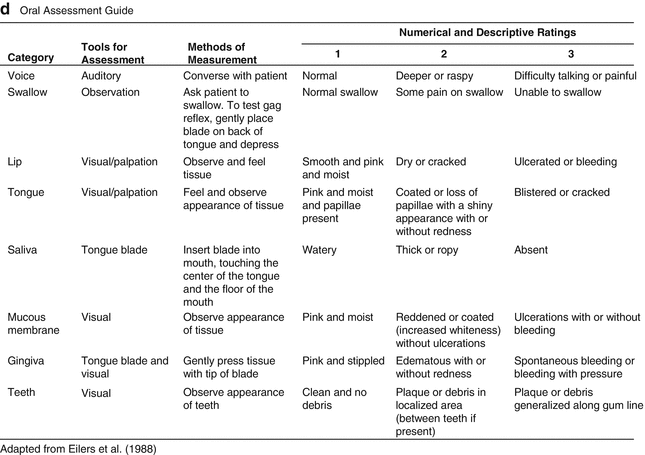


Fig. 11.2
Oral Mucositis Grading Scales. (a) World Health Organization scoring criteria. (b) National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0). (c) Oral Mucositis Assessment Scale. (d) Oral Assessment Guide
The Oral Mucositis Assessment Scale (OMAS) was developed by a panel of experts to be an objective, simple, and reproducible assessment tool to be applied to multicenter clinical trials of mucositis (Sonis et al. 1999). The OMAS measures degree of ulceration, pseudomembrane formation, and mucosal erythema in specific mouth sites (Sonis et al. 1999) (Fig. 11.2). Testing has demonstrated that the OMAS is reliable and valid in adults with the only reported limitation being that patients with severe mucositis had difficulty undergoing repeated examinations (Sonis et al. 1999). Sung et al. (2007) demonstrated that the OMAS is valid in the measurement of mucositis in children ≥6 years of age.
An example of a combined scale that includes both objective and subjective experiences as well as functional dimensions is the Oral Assessment Guide (OAG) (Eilers et al. 1988). The OAG was designed primarily as a clinical tool and is useful for recording the condition of the oral cavity (Eilers et al. 1988). The OAG consists of numerical and descriptive ratings in eight categories including voice, swallowing, lips, tongue, saliva, mucous membranes, gingivae and teeth. Each descriptive category is assessed on a numeric scale from 1 to 3 with the overall oral assessment score the sum of the eight categories (Eilers et al. 1988) (Fig. 11.2). Chen et al. (2004) used the OAG to assess children with leukemia and lymphoma undergoing chemotherapy and were able to demonstrate content validity of the instrument.
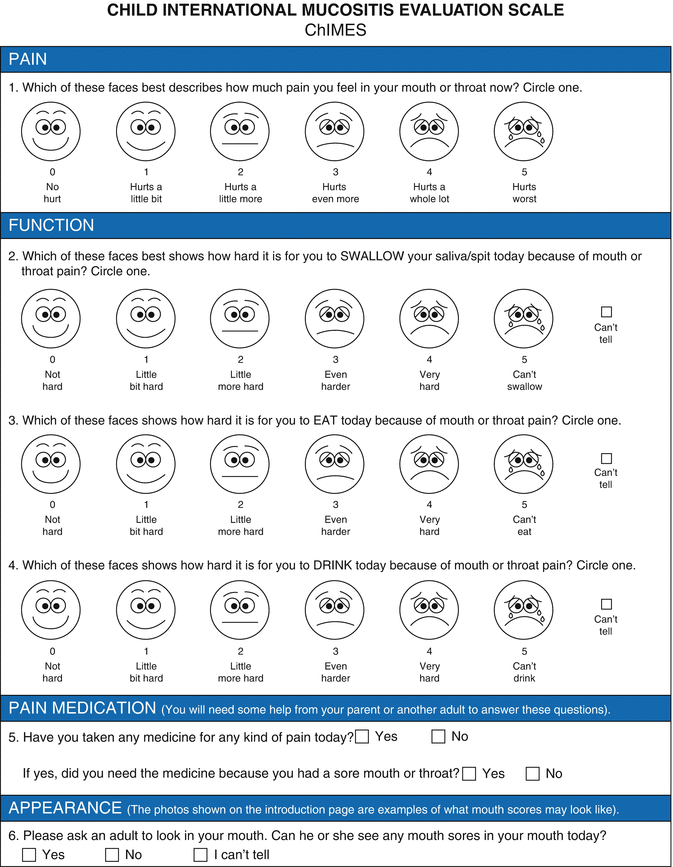
Tomlinson et al. (2008a, b, c) identified four main areas of concern from the existing literature in mucositis assessment and tool development in the pediatric oncology population: (1) the challenge of oral assessment in children with relation to age and cooperation; (2) the need for proxy responses, while recognizing the challenges of reporting pain and function attributed to oral mucositis; (3) the need for an instrument that is simple, quick to complete and easy to use in almost all children; and (4) educational considerations. To address these concerns, their group developed the Children’s International Mucositis Evaluation Scale (ChIMES; Fig. 11.3), an assessment instrument for both child self-report and parent proxy-report (Tomlinson et al. 2009). ChIMES assesses three main areas: (1) pain (i.e., pain assessment, amount of pain medication received), (2) function (i.e., effect on eating, drooling/pooling of saliva, effect on drinking), and (3) appearance (i.e., presence of ulcers) (Tomlinson et al. 2009). ChIMES has been shown to be understandable, with content validity and acceptability, and has been prospectively validated in a multicenter cohort of children receiving chemotherapy and after HSCT (Tomlinson et al. 2009, 2011; Jacobs et al. 2013).
11.4 Prevention and Treatment of Oral Mucositis
In recent years there has been an increase in research regarding the prevention and treatment of mucositis. However, evidence-based interventions remain limited, especially in pediatric patients. Generally, the clinical focus continues to be on palliation of the symptoms of mucositis (Eilers and Million 2011). In a Cochrane review, Clarkson et al. (2010) showed that there is limited evidence that low-level laser therapy (LLLT) may be beneficial in the treatment of OM. In a separate Cochrane analysis Worthington et al. (2011) reported that ten agents showed potential benefit in OM prophylaxis; these agents were reviewed by Eilers and Million (2011) and are summarized in Table 11.2.
Table 11.2
Prevention and treatment of oral mucositis
Intervention | Level of support | Population studied | Gradea |
|---|---|---|---|
Prevention | |||
Aloe Vera | Weak unreliable evidence the solution was beneficial for the prevention of moderate to severe mucositis | Head and neck cancer receiving radiotherapy | 2C |
Amifostine | Weak unreliable evidence from 11 low quality trials that amifostine is beneficial for the prevention of any mucositis | Combinations of head and neck cancer, other solid tumors and hematologic malignancies receiving radiotherapy, stem cell transplant, non-myeloablative chemotherapy or a combination | 2C |
Antibiotic (polymyxin/tobramycin/amphotericin [PTA])—lozenges/paste | Weak unreliable evidence that PTA lozenges may be beneficial for the prevention of any mucositis | Head and neck cancer receiving radiotherapy | 2C |
Cryotherapy | Found to be beneficial in the prevention of all the outcome categories of mucositis—any, moderate and severe mucositis | Hematologic malignancies with chemotherapy or stem cell transplantation | 2B |
Glutamine | Weak evidence that glutamine is beneficial for the prevention of severe mucositis | Combinations of head and neck cancer, other solid tumors and hematologic malignancies receiving radiotherapy, stem cell transplant, non-myeloablative chemotherapy or a combination | 2B |
Granulocyte colony-stimulating factor (G-CSF) | Weak unreliable evidence that G-CSF is effective for the prevention of severe mucositis | Combinations of head and neck cancer, other solid tumors and hematologic malignancies receiving radiotherapy, stem cell transplant, non-myeloablative chemotherapy or a combination | 2C |
Honey | Weak unreliable evidence with substantial heterogeneity that honey may be beneficial in the prevention of any mucositis | Head and neck cancer receiving radiotherapy | 2C |
Palifermin (keratinocyte growth factor) | Found beneficial for the prevention of all outcome categories of mucositis, moderate mucositis and severe mucositis | Combinations of head and neck cancer, other solid tumors and hematologic malignancies receiving radiotherapy, stem cell transplant, non-myeloablative chemotherapy or a combination | 2B |
Low-level laser therapy | Weak unreliable evidence that laser is beneficial for the prevention of severe mucositis | Combinations of head and neck cancer, other solid tumors and hematologic malignancies receiving radiotherapy, stem cell transplant, non-myeloablative chemotherapy or a combination | 2B |
Sucralfate | Evidence that sucralfate is effective in the prevention of severe mucositis, with a 33 % reduction in severe mucositis in treatment group compared to placebo | Mostly head and neck cancer receiving radiotherapy, some trials with participants with other cancer types | 2B |
Treatment | |||
Low-level laser therapy | Limited evidence that low-level laser is beneficial in reducing the severity of oral mucositis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||