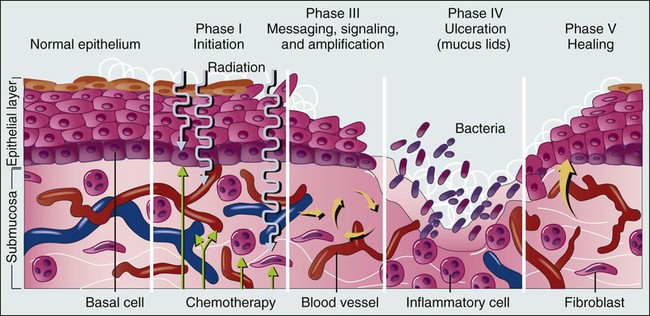
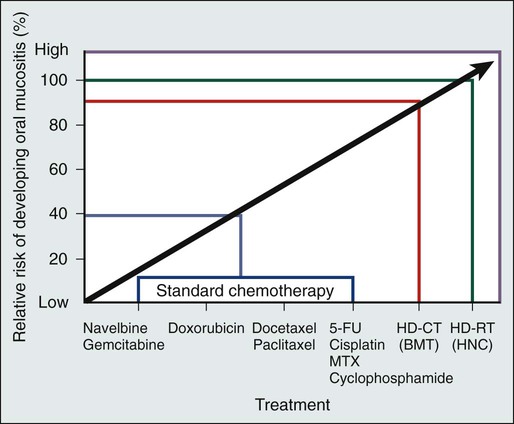
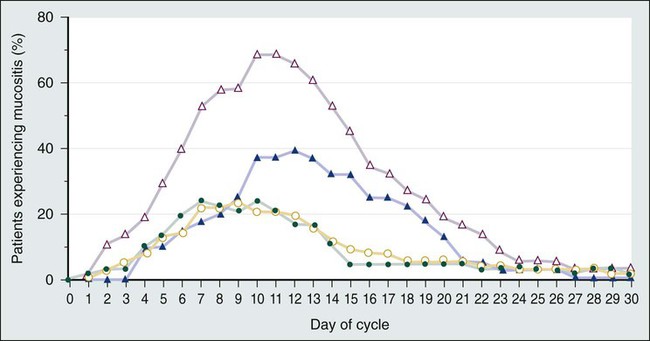
Kostandinos Sideras, Christopher L. Hallemeier and Charles L. Loprinzi • Mucositis, a major dose-limiting toxic effect of chemotherapy for solid tumors, develops in 5% to 40% of patients. • Mucositis develops in 70% to 100% of patients who receive high doses of chemotherapy with bone marrow rescue. • Mucositis is the most troublesome acute reaction for patients undergoing radiation therapy directed at the oral cavity. • Radiation therapy directed at the oral cavity frequently causes a host of other oral complications including xerostomia, dental caries, tissue necrosis, and taste alterations. • Oxidative stress caused by cytotoxic chemotherapy and radiation therapy leads to upregulation and subsequently amplification of multiple inflammatory pathways in a complex process. This subsequently leads to mucosal ulceration. • Secondary infections occur as a result of treatment-induced immunosuppression. • The importance of instituting oral hygiene protocols in patients receiving chemotherapy is well established. • Cryotherapy is the most conventional and easy to use preventative method, at least for 5-fluorouracil–based bolus therapy, and it appears to have implications for other chemotherapeutic regimens as well, such as edatrexate and high-dose melphalan therapy. • Keratinocyte growth factor has been approved by the Food and Drug Administration for use with high-dose chemotherapeutic regimens associated with high rates of mucositis and has shown promise in other settings as well. • Low-level laser therapy has shown promise, but its use is limited for now to centers that are able to support its use. • Pretreatment dental care, good oral hygiene, and sophisticated treatment planning is recommended for patients receiving radiation therapy. • Overall, evidence is lacking regarding the efficacy of various agents in promoting healing of the oral mucosa after mucositis is established. • Systemic analgesic therapy of mucositis pain with narcotic medications is well established and recommended. • Antibiotics and/or antifungal medications should be given to patients with evidence of infection. • In the palliative setting, various mouthwashes are widely used in clinical practice based on provider preference and experience. These mouthwashes most frequently contain combinations of diphenhydramine, viscous lidocaine, magnesium hydroxide/aluminum hydroxide, nystatin, and corticosteroids. The efficacy of these measures has not been adequately evaluated to date. • Baking soda mouth rinses appear to be the most economical solution, although efficacy is not clearly established. • Low-level laser therapy has also shown promise in treating established mucositis lesions. The oral cavity is a common site for chemotherapy- and radiation-induced toxicity. Manifestations of this toxicity include alimentary tract mucositis, secondary infectious complications induced by bacteria, fungi, and viruses, and graft-versus-host disease in patients receiving allogeneic bone marrow transplants. Although alimentary tract mucositis can involve the entire gastrointestinal tract,1 it is most frequently manifested in the oral cavity as ulceration, pain, and bleeding. Mucositis leads to significant patient morbidity and a decline in quality of life, and it limits the use of additional chemotherapeutic treatment. Moreover, the economic burden of this frequent oncologic complication is also considerable.2,3 This chapter discusses the etiology, incidence, risk factors, prevention, and treatment of oral toxic effects of standard chemotherapy, intensive marrow-ablative chemotherapy, and radiation therapy. The prevention and treatment of graft-versus-host disease is beyond the scope of this chapter. It is currently accepted that the process of mucosal injury and subsequent healing is not limited to the epithelium alone but involves all layers of the mucosa, including the extracellular matrix. A five-stage process has been postulated to explain the complex molecular, cellular, and histologic events associated with chemotherapy-induced mucosal injury (Fig. 43-1).4 Oxidative stress related to chemotherapy is thought to be responsible for the first phase of mucosal injury (the initiation phase). The second phase involves the upregulation of transcription factors and the generation of messenger signals (primary damage response phase). In this stage, upregulation of nuclear factor (NF)–κB is thought to play an important role in the subsequent upregulation of multiple proinflammatory cytokines, such as tumor necrosis factor (TNF)–α, interleukin (IL)-1b, and IL-6. NF-κB is also thought to upregulate cyclooxygenase-2, which in turn is implicated in the upregulation of matrix metalloproteinase.5 In addition, activation of the ceramide pathway, fibronectin breakup, and macrophage activation are other complex events that take place, leading to further mucosal injury and apoptosis. The third phase of mucosal injury involves additional signaling and amplification of the aforementioned pathways through biological feedback, leading to the generation of additional proinflammatory cytokines (the signaling and amplification phase). Until this point the biologically altered mucosa appears anatomically intact, but epithelial proliferation halts. The fourth phase consists of the symptomatic phase of mucositis, involving erythema, mucosal ulceration, plaque formation, pain, and bleeding (the ulceration phase). Immune cell infiltration occurs in this phase. Microbial superinfection and reduction in salivary gland function can complicate and amplify the mucosal injury. Oral candidiasis and herpes simplex virus (HSV) infections are particularly common, constituting the majority of the infectious complications seen in patients with mucositis, although the increased risk of bacteremia in patients with mucositis also points to the role of bacterial superinfections of the ulcerative lesions. It is important to note that although microbial superinfections can influence the duration and severity of mucositis, they are not believed to be involved in the pathogenesis of mucositis. This belief is supported by multiple studies that have failed to show any benefit of antimicrobial agents in the prevention of mucositis. The fifth and final phase involves the healing of the mucosa, a process that depends on angiogenesis and increased biological activity in the extracellular matrix (the healing phase). In patients undergoing myeloablative chemotherapy, the healing phase may not begin until leukocyte recovery. It is important to understand that these phases do not necessarily follow a linear progression but may occur simultaneously at different locations.6 The type of chemotherapeutic agents used, the specific dose, route, and frequency of administration, and whether the chemotherapy is given as monotherapy or in combination with other agents and modalities of treatment significantly affects the degree of mucosal injury (Fig. 43-2). Regarding the time course of mucosal injury, 5-FU–induced mucositis is usually first noticed from 3 to 7 days after initiation of therapy. Incidence peaks at 7 to 12 days and diminishes by around 2 to 3 weeks (Fig. 43-3). With myeloablative chemotherapy for hematologic malignancies, mucositis severity can peak up to 18 days after initiation of therapy.8 Chemotherapy for the treatment of solid tumors leads to the development of mucositis in 5% to 40% of patients (5% to 15% for patients with grade 3-4 mucositis).9 Many modern treatment approaches are still associated with this problem and, because of their aggressiveness, may be more toxic.10 A vast experience exists with 5-FU, the drug most commonly used to treat gastrointestinal malignancies. Although it has long been believed that continuous administration of 5-FU carries a higher risk of mucositis than does bolus administration, a metaanalysis of trials failed to support this association.11 When myeloablative chemotherapy is used, the incidence of mucositis increases to 70% to 100% (21% to 67% for patients with grade 3-4 mucositis).8,12 The increase in the incidence of mucositis appears to be especially true for induction regimens containing high-dose melphalan.8 When chemotherapy is used in combination with radiation therapy to treat cancer of the head and neck, the mucositis rate approaches 90% to 100% (43% for patients with grade 3-4 mucositis).13 The role of age and gender in the development of mucositis has not yet been clearly defined.14 Evidence is also conflicting about whether the type of bone marrow transplant (autologous vs. allogeneic), the use of total body irradiation in the conditioning regimen, the use of granulocyte colony-stimulating factor (G-CSF), or the baseline nutritional status are related to the risk of mucositis.14 On the other hand, poor oral hygiene, dental caries, periodontal disease, high titers of HSV, and positive cultures for Candida tropicalis are generally accepted to be risk factors.14 Finally, interpersonal variability in the development of mucositis has been observed for years. This variability may be due to the differences in metabolism of the chemotherapeutic drugs from person to person. A prime example is the drug methotrexate, which results in much higher degrees of mucositis in patients with an inability to metabolize this drug. Another example involves polymorphisms in the genes for the glutathione-S-transferases, which are important detoxification enzymes. Patients with reduced activity of these enzymes have nearly double the risk of developing mucositis.15–16 Multiple targeted therapies, such as epidermal growth factor receptor (EGFR) inhibitors, vascular endothelial growth factor inhibitors, mammalian target of rapamycin inhibitors, and other drugs have emerged in the treatment of various human cancers, either as part of standard clinical practice or as part of investigational clinical studies. These newer therapies can also have adverse oral effects. However, oral complications with targeted therapies are less frequent and generally milder and only rarely lead to treatment discontinuation. Mucosal ulcerations appear to be more consistent with aphthous ulcers than frank mucositis.17 The mammalian target of rapamycin inhibitors are perhaps the targeted therapies with the highest incidence of mucositis. Everolimus and temsirolimus are reported to produce a 20% to 40% incidence of grade 1 to 2 mucositis and a 3% to 4% incidence of aphthous ulcerations but only a 1% to 3% incidence of grade 3 complications.18 As single agents, the EGFR inhibitors panitumumab and erlotinib, known primarily for causing a characteristic skin rash, are responsible for approximately a 20% incidence of grade 1 to 2 mucositis, but only 1% of patients experience grade 3 mucositis or require treatment discontinuation for this reason.18 Mucosal inflammation without overt mucositis has been reported in 14% of patients with everolimus. With regard to other tyrosine kinase inhibitors, imatinib and sunitinib have been reported to cause between a 10% and 38% incidence of mucositis, but only 2% to 3% of patients have mucositis of grade 3 or higher.18 Oral adverse effects with drugs such as bevacizumab, trastuzumab, or lapatinib are very rare. It should be kept in mind that underreporting of oral adverse effects of biological therapies has been identified as a possible reason for the low incidence reported in some trials. When these newer therapies are combined with traditional chemotherapeutics, they can increase the usual severity of the individual chemotherapeutic drugs. Although some major studies have failed to reveal an increase in the incidence of mucositis when EGFR inhibitors are combined with radiation,19 a metaanalysis has shown that there is indeed such an increased risk (hazard ratio 1.76, P < .001).20 Although evidence is insufficient to support a particular approach,21 the institution of comprehensive oral care protocols for patients receiving chemotherapy for solid tumors is generally recommended.22,23 Multiple oral care protocols have demonstrated feasibility and tolerability, and some have produced a reduction in the severity of mucositis and an improvement in patients’ ability to cope with their symptoms.24 Such oral care protocols are usually implemented by nursing staff and involve various degrees of patient education. These protocols can include cavity screening and dental consultations, basic oral care with soft tooth brushing, flushing, and rinsing, regular inspection of the oral cavity, and avoidance of smoking, alcohol, and spices.25,26 The cross-study differences seen in two similar treatment arms illustrated in Figure 43-3 may be related to the use of nurse-directed oral care recommendations in the second study that were not used in the first study. Regarding the oral care of candidates for myeloablative chemotherapy, current guidelines that were drafted in 2009 by multiple organizations in a global effort recommend a formal dental evaluation and performance of any needed dental work before institution of conditioning regimens.27,28 This dental work includes appropriate treatment of caries, proper fitting of dental prostheses, and extraction of teeth with significant periodontal disease. These interventions should ideally be performed 10 to 14 days before any conditioning therapy. During therapy, oral hygiene should be maintained; rinses should be performed four to six times a day with use of either sterile water, normal saline solution, or sodium bicarbonate solutions, and patients should brush their teeth at least twice daily with a soft or ultra-soft toothbrush or a Toothette (i.e., a foam swab on a stick). Use of toothpaste is optional, and daily dental flossing should be performed by patients experienced in the technique, if it can be done without trauma. Orthodontic appliances and space maintainers can be removed during therapy, although, if good tissue integrity and satisfactory daily oral hygiene is maintained, their use can continue during the initial conditioning phase.27 In a retrospective review of 140 patients undergoing autologous bone marrow transplantation at a single institution, patients received professional oral health care after the initiation of local institutional guidelines in 2005. Patients treated after the institution of these guidelines (2005 to 2009) had a 66% incidence of mucositis compared with a 93% incidence for patients who did not receive professional oral health care in prior years (2002 to 2005).29 In the past, microorganisms were hypothesized to play a central role in the pathogenesis of mucositis. This hypothesis is no longer believed to be true, in part because both topical and systemic antibiotics have failed to significantly change the incidence and severity of mucositis, and as a result, they are currently not recommended for routine use.21,23,30 One explanation for the general failure of antimicrobial agents in this setting may be their inability to significantly eradicate microbes from the oral cavity, or the possibility that alterations in the microbial flora by antimicrobial agents might cause more harm than good.30,31 Another explanation is that microorganisms probably play a more complex and possibly a lesser role than initially thought. It is currently suggested that instead of being involved in the initiation phase, microorganisms probably intensify the inflammatory process associated with the later phases of mucosal injury.4 However, an association exists between the severity of mucositis and the incidence of sepsis, which suggests that microbes may use the already damaged mucosa as a portal of entry. Of all the antiseptics, chlorhexidine has been most extensively studied, with the results of nine randomized controlled trials being mixed. All of these trials ultimately provide no evidence for the benefit of chlorhexidine, and as a result, its use is not recommended.23–23 Iseganan (a naturally occurring peptide with broad antimicrobial spectrum) and a topical povidone-iodine preparation have also failed to show benefit after each compound was tested in two randomized clinical trials.21 Reactivation of HSV, which frequently manifests in patients as oral ulceration, can be a significant complication for patients receiving myeloablative chemotherapy. Nonetheless, HSV is thought to have a marginal role in causing frank oral mucositis.24 HSV infection should be suspected if mucositis persists or appears to worsen 2 or more weeks after transplantation. Guidelines for testing, prophylaxis, and treatment of HSV infection are well established.27 Oral cryotherapy during administration of chemotherapy is hypothesized to work by cooling oral mucosal tissues and thereby causing local vasoconstriction during periods of peak chemotherapy blood concentration, thus decreasing the delivery chemotherapy to the oral mucosa. It is currently recommended in three specific clinical settings and is considered to be investigational in several others.23–23 A North Central Cancer Treatment Group randomized clinical trial demonstrated that oral cryotherapy could inhibit the development of bolus 5-FU–induced mucositis.32 This result has been independently validated by multiple other investigators.33–36 The therapy is administered by having the patient suck on crushed ice, starting 5 minutes before 5-FU administration and continuing for a total of 30 minutes. Longer duration of oral cryotherapy (60 minutes) does not appear to provide any additional benefit in this setting.37 Four small nonrandomized phase 1 and 2 trials have used 20 to 30 minutes of oral cryotherapy for prevention of mucositis in patients receiving edatrexate, a methotrexate analogue with improved preclinical antitumor activity. Three out of the four trials have shown good tolerability of edatrexate when used with oral cryotherapy,40–40 with one study showing high toxicity despite this preventative strategy.41 Current guidelines recommend cryotherapy as an attempt to decrease mucositis in patients treated with bolus edatrexate.23–23 At least five small prospective, nonrandomized studies in patients receiving high-dose melphalan therapy have tested the efficacy of oral cryotherapy compared with historic control subjects. Grade 3 mucositis developed in only 0% to 11% of patients treated with oral cryotherapy compared with a greater than 70% incidence of mucositis in historic control subjects.42–46 In the only randomized, placebo-controlled study, in which room-temperature normal saline solution was used as a placebo, 40 patients were treated with cryotherapy or placebo for 30 minutes before and 6 hours after chemotherapy with high-dose melphalan. Grade 3 mucositis was experienced in 14% of patients receiving cryotherapy compared with 74% of patients receiving normal saline solution.47 Although the need for such prolonged administration of cryotherapy is questionable because of patient noncompliance and the probably equivalent efficacy of shorter administration, cryotherapy appears to be a promising strategy in lowering mucositis in patients receiving high-dose melphalan therapy, and it is currently recommended in this setting.22 One randomized study in patients receiving methotrexate did not show a benefit.48 Overall, these data point to a possible role for cryotherapy in more diverse settings than previously thought. In general, insufficient evidence exists regarding the effectiveness of antioxidant compounds, such as all-trans retinoic acid and vitamin E, to prevent oral mucositis in patients receiving chemotherapy.14 Insufficient evidence also exists to support the prophylactic use of propantheline, an anticholinergic drug that is thought to reduce the amount of etoposide secreted into the saliva.21 Regarding sucralfate, a coating agent, at least nine randomized clinical studies have provided mixed results. However, the most recent metaanalysis suggests a 33% reduction in severe mucositis with this agent.21 Most of the studies are in patients treated with radiation therapy. Nonetheless, sucralfate is not currently recommended by any set of guidelines.22,23 Because inflammatory mediators appear to play a central role in mucositis development, the use of antiinflammatory agents has been proposed as a method to prevent mucositis. Pentoxifylline, a TNF-α and IL-2 inhibitor, misoprostol (an analog of prostaglandin-E1), and prostaglandin-E2 have failed to show benefit in randomized controlled trials.21 Glutamine is a nitrogen-rich nonessential amino acid with a critical role in nucleotide synthesis, muscle function, and overall metabolic homeostasis. However, during periods of stress it becomes a conditionally essential amino acid, and its stores can be significantly depleted, as is the case in patients with cancer. Multiple trials to date have attempted to investigate the beneficial potential of different glutamine preparations through both the parenteral and oral routes, with mixed results. Glutamine had been administered parenterally, as part of total parenteral nutrition, or as an IV infusion mixed with normal saline solution. It has also been administered as an oral supplement and in “swish and swallow” mouthwash preparations. Overall, these trials have been small, and conclusions have been difficult to draw; in the transplant setting, the possibility of harm has been raised.21,49 None of these preparations is currently recommended, but further research is ongoing.22,23 An oral suspension form of L-glutamine (AES-14) was shown in a randomized controlled study of 326 patients with breast cancer who were undergoing chemotherapy to reduce the rate of grade 2 and higher oral mucositis by 11% (50% to 39%: P = .03) and grade 3 and higher mucositis by 5% (1.2% vs. 6.7%; P = .005).50 Growth factors, systemically or topically, are hypothesized to prevent oral mucositis because of their potential to improve healing. Although the use of subcutaneous G-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF) has been associated with reduced mucositis in certain randomized trials, the data are inconclusive.23–23 However, one large study randomly assigned patients to prophylactic G-CSF or placebo; this study involved 195 patients undergoing chemotherapy with cyclophosphamide, doxorubicin, and etoposide for small-cell lung cancer.51 Patients treated with G-CSF had a significantly lower rate of oral mucositis (70% vs. 53%). In addition, a study of adjuvant Taxotere (docetaxel), Adriamycin (doxorubicin), and cyclophosphamide (TAC) had to be amended after 116 patients with breast cancer were enrolled because of the high incidence of febrile neutropenia. As a result, 423 additional patients who were treated with TAC received prophylactic G-CSF. The addition of G-CSF, along with reducing the rate of febrile neutropenia, was also associated with a reduced rate of grade 2 or higher stomatitis, from 32% to 23% (P = 0.01).52 In conclusion, subcutaneous injections of G-CSF appear to reduce the rates of oral mucositis. However, this intervention is not recommended for mucositis reduction alone, and its use is restricted to patients with currently approved indications, such as high risk of neutropenic fever. Topical keratinocyte growth factor (KGF), which is secreted by injured mucosal epithelium, was hypothesized to have efficacy in mucositis prevention. One such preparation, Palifermin, has been approved by the Food and Drug Administration (FDA) for use in preventing mucositis induced by myeloablative chemotherapy. The mechanism of action of KGF is thought to be complex, with effects both on stimulation of epithelial proliferation and antiinflammatory properties.53 The recommendation for approval was based on a study of 212 patients randomly assigned to receive intravenous (IV) KGF or placebo for 3 consecutive days immediately before the initiation of conditioning therapy.54 Grade 3 or 4 mucositis developed in 63% of patients in the KGF group and in 98% of patients in the placebo group. KGF has been tested in at least five more trials.21 Two of these studies were in patients undergoing myeloablative chemotherapy, two were in patients with solid tumors (colorectal cancer and sarcoma), and one was in patients undergoing chemoradiation for head and neck cancer. In all trials except for the last (where a weekly instead of a daily dose was used), a benefit has been suggested.21 KGF is currently recommended for patients undergoing myeloablative chemotherapy but not in patients being treated for solid tumors, because further studies are needed.22,23 Although effective, its use constitutes an expensive option for mucositis prevention. Accumulating evidence suggests that low-level laser therapy (LLIT) has the ability to promote wound healing and reduce pain and inflammation. Several trials have suggested a benefit for laser therapy for the prevention of oral mucositis. A recent metaanalysis of randomized trials found 11 randomized studies, 9 of which were in patients undergoing chemotherapy for solid tumors or hematologic malignancies.55 The results of these trials have been impressive, with a relative risk reduction of developing mucositis of 2.03 (95% confidence interval [CI], 1.11 to 3.69), a shortening of the duration of grade 2 or higher mucositis by 4.38 days (95% CI, 3.35 to 5.40), and significant reductions in mucositis severity and pain.55 A Cochrane review included five of these trials and also suggested significant benefit in mucositis prevention.21 However, the expense and the need for specialized training and equipment limit the widespread applicability of this approach. Guidelines recommend the use of this technology in specialized centers that have the appropriate expertise; further studies are needed.22,23 However, it is true that the expense and availability of the technology is significantly improving and the technical specifications are being standardized, and thus it is possible that widespread clinical use may soon become a reality. Amifostine, a cytoprotective agent, has been primarily tested in patients undergoing head and neck chemoradiation. However, in at least three studies, amifostine has been tested in patients undergoing high-dose chemotherapy or myeloablative chemotherapy with positive results.21 Although the newer subcutaneous formulation appears to address the toxicity and administration difficulties associated with daily intravenously administered amifostine, further studies are needed. An oral spray of intestinal trefoil factor, a peptide secreted by goblet cells and involved in mucosal protection, was tested in one randomized study of 99 patients undergoing 5-FU–based chemotherapy for colorectal cancer. A 75% to 81% reduction in the risk of mucositis was found, and further studies with the agent are under way.56 In two studies in patients undergoing bone marrow transplantation, a benefit has been suggested for a neutral supersaturated calcium phosphate rinse (Caphosol).57,58 Allopurinol mouthwashes, despite constituting “standard clinical practice” at some institutions in the early 1990s, are currently not recommended because several randomized clinical trials have convincingly shown that they have no benefit.21,59,60 Traumeel S, a homeopathic remedy, and chamomile mouthwash have also failed to show any benefit. Scant information is available regarding the effective treatment of chemotherapy-induced mucositis, despite a plethora of prescribed remedies. Hence initial treatment of established mucositis varies significantly among institutions, and different providers often prescribe remedies based on their individual experience and preference.61 Among the many proposed treatments are various mouthwashes, coating agents, topical anesthetics or analgesics, antiinflammatory agents, systemic narcotics, and topical and systemic growth factors.24 These treatments are aimed at promoting the healing of the injured oral mucosa and thus limiting the severity and duration of ulcerations, as well as palliating the symptoms of oral mucositis. For patients with established mucositis, one of the first therapeutic measures frequently used consists of having patients rinse their mouths every 2 to 4 hours with a salt and baking soda solution (½ tsp salt plus ½ tsp baking soda in an 8-oz glass of warm water). This rinse is often soothing and is thought to be cleansing. Some centers use baking soda alone, because the addition of salt is thought to be too drying to the mucosa. In one multicenter study, 200 patients receiving standard chemotherapy who followed a carefully planned oral hygiene protocol (PRO-SELF) were randomly assigned to one of three different mouthwashes; salt and soda, chlorhexidine, or “magic” mouthwash (lidocaine, Benadryl, and Maalox). No significant differences were observed in time to cessation of the signs and symptoms of mucositis among the three regimens, with salt and soda being the least costly.62 Mouthwashes, often referred to as “magic” or “miracle mouthwash,” are topical preparations of analgesic, anesthetic, and coating agents, the composition of which varies across institutions. The most common ingredients are diphenhydramine, viscous lidocaine, magnesium hydroxide/aluminum hydroxide, nystatin, and corticosteroids.63 Other ingredients include benzocaine, milk of magnesia, chlorhexidine, kaolin, and pectin.24 Despite their widespread use, the general lack of evidence supporting their efficacy and tolerability does not support the inclusion of any of these preparations in formal guidelines.22,23 Further study of these palliative mixtures, however, is strongly encouraged given their overall availability, ease of administration, frequent use, and low cost. Chlorhexidine mouth washes are not recommended, because studies have failed to support the use of this agent for the treatment of established mucositis.22,23 Coating agents such as sucralfate have also failed to show any benefit for the treatment of established chemotherapy-induced mucositis when tested in small randomized trials.64 Other coating agents include Gelclair and MuGard, which are oral lubricating gels and are used by some institutions based on anecdotal reports. No well-conducted trials exist, and Gelclair did not show promise in small preliminary studies.65,66 Oral mucositis pain can be severe and significantly interfere with the quality of life of patients receiving chemotherapy. Management should follow the same aggressive guidelines used to treat pain in patients with cancer in general.68 The use of patient-controlled anesthesia is effective and well established for treating mucositis pain, especially in the hematopoietic stem cell transplantation setting. Although it is not superior to continuous infusion delivery in controlling pain, the use of patient-controlled anesthesia allows for a lesser quantity of narcotics to be used.64 Low-level laser therapy (LLLT) has also shown promise in treating established mucositis lesions.64 In a randomized trial, 37 patients with hematologic malignancies who were undergoing myeloablative chemotherapy and who had established grade 1 or 2 oral mucositis were randomly assigned to LLLT or sham illumination.69 Progression to grade 3 mucositis occurred in 16 of 18 sham-treated patients and in only 3 of 16 laser-treated patients. In another study, 21 children and adolescents undergoing high-dose chemotherapy were randomly assigned to laser therapy versus sham treatment.70 Patients were treated for 5 consecutive days after mucositis was first diagnosed. After 7 days, only 1 of 9 patients in the treatment group had oral lesions versus 9 of 12 patients in the sham group. As discussed in the prevention section, this technology is awaiting further development.
Oral Complications
Introduction
Pathophysiology of Mucosal Injury and Clinical Manifestations
Oral Complications From Chemotherapy, Including Myeloablative Chemotherapy
Incidence and Risk Factors
Biological Therapies
Oral Care Protocols and Oral Hygiene
Antimicrobial and Antiseptic Interventions
Cryotherapy
5-FU–Based Chemotherapy
Edatrexate
High-Dose Melphalan
Antioxidants, Anticholinergics, and Coating Agents
Antiinflammatory Agents
Amino Acids
Growth Factors
Low-Level Laser Therapy
Other Interventions
Treatment of Chemotherapy-Induced Oral Mucositis
Mouthwashes and Coating Agents
Systemic Analgesics
Laser Therapy
Oncohema Key
Fastest Oncology & Hematology Insight Engine