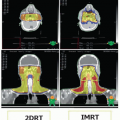
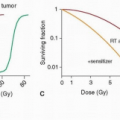
Oral and Maxillofacial Rehabilitation of Patients with Head and Neck Cancer
Richard C. Cardoso
Theresa M. Hofstede
Patricia C. Montgomery
Jack W. Martin
Adam S. Garden
Ruth A. Aponte-Wesson
Alexander M. Won
Mark S. Chambers
INTRODUCTION
Optimal care of patients with cancer of the head and neck requires the combined efforts of a team of health care providers whose collective goal is not only to cure patients of malignant disease but also ensure the well-being and maintain quality of life of patients during and after treatment. In most patients with cancer, challenges seen in the oral cavity mirror those of the general population—moderate to advanced periodontal disease, dental caries, poorly restored dentition, dental and soft tissue pathologies associated with the use of tobacco and alcohol, poor nutritional status, general hygiene neglect, or a combination of these factors.1,2 Evaluation, treatment, and prevention of any preexisting oral and dental disease are important aspects of the overall treatment outcome for patients with cancer.1,2 Patients undergoing aggressive anticancer treatment encounter treatable, if not preventable, oral mucosal and dental sequelae that, if left untreated, could produce morbid events.3 Complications vary with each patient, depending on the individual’s oral and dental status, the type and location of malignancy, and the therapeutic approach.4 Common and frequent treatment-limiting factors, such as mucositis, infection, and bleeding, can be minimized and, in some cases, eliminated, if evaluated and treated early by an involved, trained oral oncology team. Early dental intervention can also decrease risk factors for oral complications [e.g., osteoradionecrosis (ORN)]. In some cases, ablated structures can be replaced immediately with prostheses that restore function and esthetics thereby maintaining quality of life.
Treating physicians have a medical, ethical, legal, and fiscal responsibility to ensure that patients receiving surgery, radiation therapy, and/or chemotherapy for cancer of the head and neck receive a thorough, systematic oral examination.1,4 For decades, physicians have placed a major emphasis on the prevention of malignancies through the identification and control of factors, such as tobacco use and alcohol consumption, which are associated with carcinogenic tissue changes.5,6,7,8,9,10 Despite a decrease in smoking, however, the incidence of squamous cell carcinoma of the oropharynx continues to increase; this is attributed to infection by human papillomavirus (HPV).11 HPV-associated cancers are thought to be more responsive to treatment, and therefore, a de-escalation of treatment has been suggested; however, the oncologic efficacy and preservation of function of this type of reduced treatment remain to be shown in prospective clinical trials.11 Patients with HPV-related disease are generally younger, and given they have more curable disease, the likelihood is that these patients will live longer and thus have a greater cumulative risk of developing long-term complications including treatment-related oral and dental problems.
Therefore, careful coordination between head and neck oncologists of all disciplines must enlist oral health experts for the assessment and delivery of preventive and rehabilitative oral care prior to the initiation of oncologic treatment. Miscommunication or lack of communication between the treating oncologists and oral health experts can contribute to posttreatment complications.12,13 Oral oncology/maxillofacial prosthodontics is the branch of dentistry specifically concerned with minimizing the oral morbidities associated with cancer therapy and includes the prosthetic rehabilitation of intraoral and extraoral structures that have been affected by disease, injury, surgery, or congenital malformations.14,15 Patients should be referred to the oral oncologist as early as possible to ensure the appropriate assessment of their oral/dental status, discussion of the prosthetic treatment options of oral/facial defects, and patient education to minimize the oral morbidities associated with cancer care. The primary head and neck oncologist can then integrate results of this evaluation into the overall treatment plan.
This chapter describes the scope and integration of oral oncology concepts into the treatment of patients with cancer as well as general and specific aspects of oral complications arising from cancer therapy and outlines practical approaches for preventing, recognizing, and treating the oral sequelae associated with chemotherapy and radiation therapy. This chapter also presents current concepts regarding oral and facial prosthetic rehabilitation and oncologic principles associated with the care of patients with cancer of the head and neck.
GENERAL CONSIDERATIONS
An oral/dental consultation before chemotherapy, radiation therapy, or head and neck surgery is extremely important in the oral management of patients with head and neck cancer.1,16,17,18,19,20,21
Oral complications associated with cancer therapy can be classified into several general types—acute (i.e., stomatitis, infection, bleeding, mucositis, and pain) and chronic (i.e., loss of function, ORN, and xerostomia).22 In most cases, preexisting conditions strongly influence the development or persistence of these complications in the oral cavity. Simple measures, such as good oral hygiene, can often minimize treatment-induced morbidities (i.e., reduce the incidence and severity of mucositis). Complications arise primarily in three anatomic sites—the mucosa, periodontium, and teeth.4
Complications arising during cancer therapy may result either from the malignant disease process itself or from treatment (e.g., complications of chemotherapy related to myelosuppression/immunosuppression, or direct cytotoxic effects of chemical agents on oral tissues). For patients receiving a tumor-ablative procedure involving the oral cavity or oropharynx, the treating oral oncologist should aim to control oral and dental complications. As part of the immediate surgical planning, the oral cavity should be prepared for appropriate prosthetic rehabilitation to correct postsurgical defects. If radiation therapy or chemotherapy or both are planned, the treating physician should be aware that the possibilities of oral complications may be increased; furthermore, the degree and type of oral sequelae may affect the patient’s compliance with or continuation of cancer treatment.23,24,25,26 Radiation therapy may directly damage oral mucosa, salivary glands, bone, and oral musculature, resulting in xerostomia, infection, ORN, trismus, dermatitis, extensive dental caries, abnormal bone development and healing, and alteration in taste acuity.1,17,27,28 Preexisting oral disease may increase the risk and severity of oral complications.17,25,29,30
An evaluation of the head and neck, an oral/dental clinical examination, and an intraoral radiographic evaluation should all be performed during the initial visit. Selected dental radiographs are essential in evaluating potential areas of infection that are not obvious on clinical examination (e.g., periapical tooth disease, interproximal caries, residual cysts, and impacted or partially erupted teeth). In addition, the oral oncologist should gather and record the patient’s history of present illness, past medical and dental history, social history, review of systems, current medications, and adverse drug reactions, as well as the anticipated cancer treatment plan. From this information, the oral oncologist can plan treatment to meet oral needs in the context of the patient’s overall status; however, treatment of malignancy must always take priority over reduction of potential complications.1,31 Complicated, laborious, and costly prosthetic restoration of teeth should be avoided until after cancer therapy is completed. The overall oral/dental goal is to remove not only current foci of infection but also potential foci of infection as this may increase the risk of ORN and other oral challenges (i.e., teeth that are nonfunctional, noncleansable, and nonrestorable).
The oral oncologist should communicate with the treating physicians to be aware of the diagnosis and staging malignancy, the goals of proposed therapy, the patient’s prognosis, the type and dose of therapy to be administered, and the timing of treatment. If radiation therapy is to be used in the head and neck area, the oral oncologist must know the volume of tissue to be radiated, the treatment schedule, the dosimetries, the fractionation scheme, the method of administration (e.g., external beam radiation therapy or brachytherapy), the type of energy (e.g., proton, photon, or electron), and the total dose to be administered.16,18,32,33,34,35,36
Oral oncologists should educate patients using tobacco, alcohol, or illicit drugs as to the harmful physical effects of these agents and explain their possible impact on oral health as well as the malignant disease process.37,38 In addition, the treatment team should immediately discourage further use of these substances and should provide information about and referrals to cessation programs. The nutritional status of patients should be assessed, and counseling should be provided, if needed, to enable patients to avoid debilitation, which may result in delayed wound healing and increased susceptibility to dental caries.27,28,39,40,41
The oral oncologist’s initial examination and early treatment of the patient are directed at documenting and managing any preexisting acute or chronic pathologic conditions, such as dental abscesses, advanced periodontal disease, dental calculus causing gingivitis, partially erupted teeth with the potential for pericoronitis, or soft tissue trauma, which may be factors in the treating physician’s selection of an overall cancer treatment strategy.1,22 Even if the planned cancer treatment is not toxic to the mucosa and is not myelosuppressive, the potential for oral complications remains. Additionally, the cancer may eventually progress, requiring prompt and aggressive therapy; for that reason, the patient’s oral and dental status should be optimized to ensure minimal predictable complications. The periodontal status and degree of tooth decay are evaluated, as are the patient’s ability and initiative to maintain optimal oral hygiene.42 Patients must modify their oral care and hygiene techniques to minimize mucosal, gingival, and dental complications that are associated with their specific treatment.42,43,44
One major objective of oral hygiene is to reduce and minimize the formation of plaque—a proteinaceous, adherent, bacteria-laden material on teeth or dental prostheses. Plaque is colonized by normal flora, which is then followed by opportunistic pathogenic organisms. Its accumulation can lead to several harmful conditions, including gingivitis (inflammation of the gingiva), which can progress to periodontal disease (the pathologic loss of supporting bone).4 Caries is another adverse condition resulting from increased caries-forming organisms and is in itself an important consideration in patients with drug- or radiation-induced xerostomia. Gross caries can result in pulpal involvement and subsequent periapical abscess formation and thus increase the risk of ORN.4,42,45
Oral surgery, definitive or intermediate direct restorations, and oral prophylactic procedures may be performed quickly and safely, if needed, under local dental anesthesia, intravenous sedation, or general anesthesia, to expeditiously remove acutely and chronically infected tissues. The oral oncologist must review the patient’s medical and hematologic status with the treating oncologist before initiating any such oral/dental treatment.46 Prophylactic periodontal procedures and scaling/root planing may be necessary before or during cancer treatment to reduce the oral contamination. Oral hygiene instructions for plaque removal should be emphasized and encouraged, including brushing twice daily with fluoride toothpaste and flossing at least once a day. Oral hygiene procedures may require modification during cancer therapy—for instance, using an extra soft-bristled toothbrush and daily use of topically applied fluoride (e.g., 0.4% stannous fluoride, 1.1% sodium fluoride, or 5,000 ppm sodium fluoride). Customized carriers for fluoride application should be constructed for patients undergoing head and neck radiation therapy and should be used throughout the remainder of the patient’s life following radiation therapy.1,46 Sodium bicarbonate or chlorhexidine gluconate mouth rinses may be beneficial to the patient in reducing microbial and fungal contamination.47 The prescribed mouth rinse should be carefully selected because some ingredients, such as alcohol and phenol, can be irritating to oral mucosal tissues.
Oral treatment plans should be designed to correct restoration with overhangs, rough or sharp edges on teeth, and any other defects likely to cause soft tissue irritation.1,48 Patients should be instructed to avoid abrasive foods likely to traumatize soft tissues. Ill-fitting intraoral prostheses should be adjusted to improve fit or not be worn during cancer therapy. Dental implants should be carefully assessed, and their removal should be considered if osseointegration is poor. The
implants should not be removed if the implant is stable or if such surgery cannot be performed within 2 weeks before the initiation of cancer therapy.49,50,51
implants should not be removed if the implant is stable or if such surgery cannot be performed within 2 weeks before the initiation of cancer therapy.49,50,51
Any potential source of oral infection should be identified and eliminated. Findings of periapical disease, severe periodontal disease, unrestorable teeth with advanced caries, supererupted teeth, and possibly unopposed dentition should be considered for extraction. Endodontic therapy is a viable alternative for infected dentition, provided that the treatment can be expeditiously performed and not delay initiation of cancer therapy.21,45 To ensure coverage of exposed bone and adequate wound healing, extractions should be performed 2 to 3 weeks before initiation of radiation therapy.24,52,53,54,55 Dental extractions should be performed as atraumatically as possible, and all sharp areas of bone should be removed. This allows for primary closure, which promotes rapid healing.21,55 ORN can occur in areas in which teeth were extracted before radiation therapy.55,56,57 Sufficient healing time is critical, particularly before radiation therapy is initiated. In general, invasive periodontal surgical procedures should be avoided due to prolonged healing time for the desired results to be achieved.
Patients receiving myelosuppressive or immunosuppressive drugs can develop posttreatment oral or systemic infections, with virus (e.g., herpes simplex virus [HSV]), fungi (e.g., candidiasis), or Gram-positive or Gram-negative microorganisms believed to have originated in the oral cavity.58,59 As a result, some oncology centers perform microbiologic cultures to assess HIV antibody titers, fungal activity, and microbes with appropriate sensitivity testing as part of the protocol for all oral-related toxicity from cancer therapy. In cases of a positive titer, use of an antiviral agent such as acyclovir, an antifungal agent such as nystatin, or an antimicrobial agent such as clindamycin is common.60,61,62
The following sections describe methods of integrating dental and oral treatment into specific oncologic therapies.
ORAL AND DENTAL ANATOMY
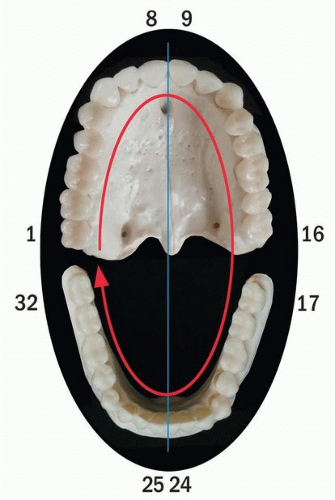
Head and neck surgeons and radiation oncologists should have a thorough knowledge of the oral and dental anatomy to enable accurate communication with the oral oncologist who is responsible for the patient’s oral care. It is important to know which teeth will be removed as part of the surgical procedure so the oral oncologist can plan and fabricate an immediate prosthesis. The universal numbering system of teeth is most often used for this purpose. In this system, teeth are sequentially numbered in the adult from 1 to 32, starting with the right maxillary third molar (No. 1), going to the left maxillary third molar (No. 16), then continuing with the opposing left mandibular third molar (No. 17), and ending with the right mandibular third molar (No. 32) (Fig. 29.1).63
Anatomic landmarks important in prosthetic rehabilitation in the edentulous maxillary and mandibular arches are shown in Figure 29.2. The stress-bearing surfaces of the edentulous maxilla for a maxillary prosthesis are the tuberosity, rugae, and palatal vault. A maxillary prosthesis is generally retained with suction; if these anatomic regions are not covered
appropriately or missing, it is difficult to retain a prosthesis. The mandibular landmarks of importance are the alveolar ridge, retromolar pad, and buccal shelf. The buccal shelf is the primary stress-bearing area for a mandibular complete denture prosthesis. It is important to cover the retromolar trigone to prevent excess resorption of the mandible. Preserving, enhancing, or reconstructing these tissues is important for support and retention of a prosthesis. Teeth that are in good periodontal condition are, of course, essential for the retention and support of the prosthesis. Conservation of the supporting tissues, irrespective of disease removal, should be a priority.64
appropriately or missing, it is difficult to retain a prosthesis. The mandibular landmarks of importance are the alveolar ridge, retromolar pad, and buccal shelf. The buccal shelf is the primary stress-bearing area for a mandibular complete denture prosthesis. It is important to cover the retromolar trigone to prevent excess resorption of the mandible. Preserving, enhancing, or reconstructing these tissues is important for support and retention of a prosthesis. Teeth that are in good periodontal condition are, of course, essential for the retention and support of the prosthesis. Conservation of the supporting tissues, irrespective of disease removal, should be a priority.64
ORAL AND DENTAL EVALUATION
Oral and dental evaluation by the head and neck oncologist should be included in the routine examination of the head and neck. The surgeon should be able to recognize acute or chronic pathologic conditions related to the dentition or supporting structures, such as advanced periodontal disease, gross dental caries, tissue irritation from poorly fitting prostheses, and poor oral hygiene1 (Fig. 29.3). Oral and dental disease should be noted by the oncologist and referred to the oral health provider for evaluation and appropriate treatment.31
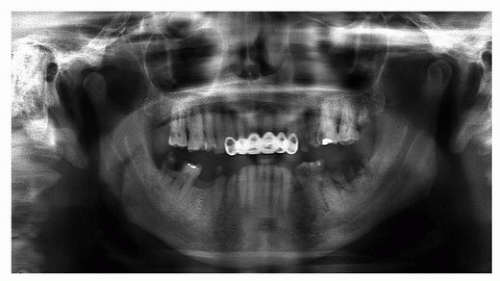
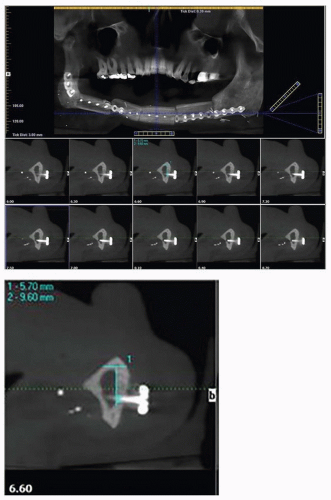
The initial oral-dental examination by the dentist will confirm any preexisting acute and chronic oral pathologic conditions (e.g., dental abscesses, teeth with advanced periodontal disease, or dental calculus causing gingivitis).1 The dental clinician should obtain appropriate diagnostic radiographs. The most common diagnostic radiographs used in an oral-dental examination are panoramic, periapical, and bite wing radiographs. A panoramic radiograph gives an overall topographic picture of the dentition, maxilla, mandible, sinuses, nasal cavity, and temporomandibular joints.65,66 These radiographs may be of diagnostic value to the treating oncologist (i.e., diagnosis of bony invasion of the maxilla or mandible by tumor) and are easily obtained in most dental offices (Fig. 29.4). Cone-beam computed tomography (CBCT) has become an invaluable tool not only in diagnosis of oral pathology but also in threedimensional planning for endosteal implants (Fig. 29.5).
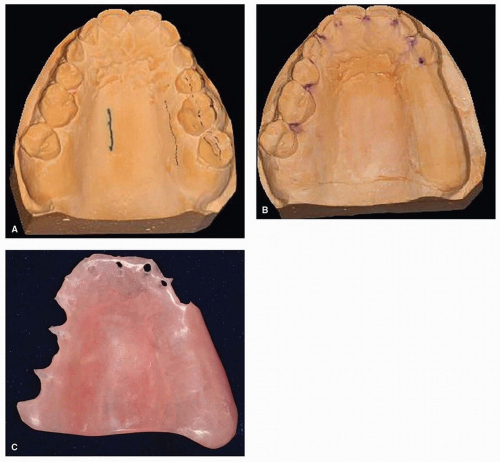
The dentition is evaluated with respect to its periodontal, restorative, and hygiene status. The oral oncologist should also determine the ability of the patient to maintain oral hygiene after the cancer treatment. Every effort should be made to eradicate any areas of infection before treatment associated with the dentition. Nonrestorable teeth can be removed at the time of the ablative surgery. Teeth should not be removed indiscriminately because of their potential to support and retain a prosthesis. Impressions of the maxilla, mandible, or external facial structures are made at the time of initial evaluation; the casts obtained from these impressions may be helpful in fabricating surgical prostheses, if needed (e.g., a surgical obturator for a patient who has undergone a maxillectomy) (Fig. 29.6). Patients are instructed on oral hygiene methods and how oral physical therapy will play an important role in the postoperative rehabilitation period. Pertinent findings associated with the oral and dental examination are then presented at the multidisciplinary treatment planning session.67,68
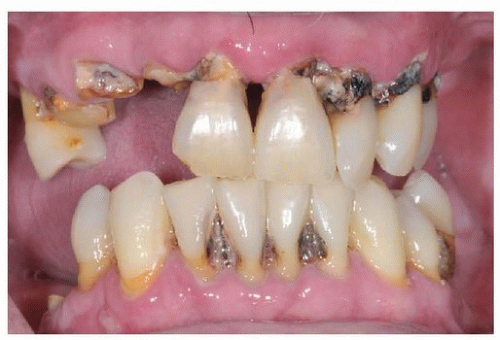 Figure 29.3. Gross caries, plaque, and calculus formation indicate poor oral hygiene and should be noted in the primary medical evaluation. Mobile teeth indicate bone loss and periodontal disease. |
Extraction of Troublesome Teeth
The following guidelines are used to identify potentially troublesome teeth for extraction prior to initiating radiation therapy to minimize the risk of ORN. Teeth within the volume of tissue to be radiated that demonstrate moderate or severe periodontal disease, advanced caries, or periapical pathologic conditions should be extracted. Also, partially impacted teeth, unopposed teeth, and teeth that would, if not extracted, oppose a segment of a resected jaw or complicate future prosthodontic rehabilitation should be extracted. In addition, healthy teeth may need to be extracted if the patient is clinically judged to be unable to maintain adequate oral hygiene following radiation therapy. Fully or deep partially impacted third molars should not be extracted due to extended healing time and risk for permanent damage to the inferior alveolar nerve. Radiologic examination, with either a panoramic radiograph or a CBCT, is the most important diagnostic tool in diagnosing periapical infection, impacted teeth, intrabony cysts, or other hard tissue disease.
Patient Education
The evaluation appointment is the ideal time to present the patient with an oral hygiene protocol designed to minimize or prevent complications associated with radiation therapy to the paranasal sinuses, parotid, nasopharynx, oral cavity, oropharyx, and/or neck. Oral hygiene procedures designed to
reduce plaque and oral flora must also be instituted. Because the effects of radiation are permanent, the patient must continue a fluoride protocol throughout his/her life.
reduce plaque and oral flora must also be instituted. Because the effects of radiation are permanent, the patient must continue a fluoride protocol throughout his/her life.
Patients who receive radiation therapy to the head and neck area involving the major salivary glands experience reduced saliva flow (i.e., salivary hypofunction or xerostomia).55 This permanent xerostomia results in dramatic increase in the susceptibility to dental caries, and the severity of the morbidity is dependent on the radiation dose, volume of tissue treated, and the age of the patient when treated.1 Therefore, patients should be placed on a fluoride regimen as numerous studies have shown that fluoride reduces radiation-induced decay of teeth if used in a systematic, predictable manner.69,70
Caries is in itself an important consideration in patients with drug- or radiation-induced xerostomia (Fig. 29.7).1,71 Reducing the potential for dental infection while maintaining optimal oral health can significantly decrease the risk of ORN in a patient who receives radiation therapy or a significant cellulitis
in a patient who receives aggressive chemotherapy.69,72,73 The surgeon should understand the importance of this concept and reinforce compliance at subsequent visits.
in a patient who receives aggressive chemotherapy.69,72,73 The surgeon should understand the importance of this concept and reinforce compliance at subsequent visits.
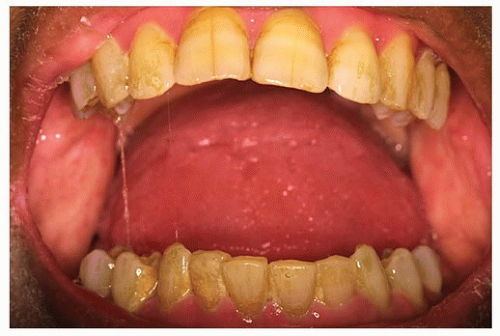 Figure 29.7. Radiation-induced xerostomia, as demonstrated by thick ropey saliva, can result in rapid onset and progression of rampant dental caries, which ultimately may result in ORN. |
Meticulous oral, dental, and prosthetic hygiene is encouraged as soon as possible after head and neck surgery.71 In general, patients are hesitant about starting oral and dental hygiene postoperatively because of a fear that the surgical site will be disturbed. Routine oral and dental hygiene can be initiated 2 weeks following surgery. In the immediate postoperative period, within a 2-week period, oral care may be limited to oral lavage and rinses.1,69,71
Interincisal opening can be challenging postoperatively, especially following a maxillectomy procedure. Postoperative physical therapy is an additional consideration in rehabilitation, and suitable techniques can be discussed and explained to the patient before surgery and reinforced postoperatively. Stretching can maintain the oral opening and allow the patient better access to the surgical defect and to the remaining oral
cavity.71,74,75 Simple opening exercises using wooden tongue blades or the fingers may be effective in restoring normal opening after surgery (Fig. 29.8A). In more complex cases, devices such as the Therabite or Dynasplint can be implemented (Fig. 29.8B).69,75
cavity.71,74,75 Simple opening exercises using wooden tongue blades or the fingers may be effective in restoring normal opening after surgery (Fig. 29.8A). In more complex cases, devices such as the Therabite or Dynasplint can be implemented (Fig. 29.8B).69,75
RADIATION THERAPY
More than 80% of patients diagnosed with cancer of the head and neck receive a course of radiation therapy as a component of their treatment.1,76 Many small or early-stage tumors of the head and neck region can be treated with definitive radiation; more advanced disease often requires a combination of therapies. If surgery is a component of the management strategy for an advanced cancer, radiation may be sequenced so that it is administered either before or after the surgical procedure. Chemotherapy is also used for patients with advanced disease and can be delivered in a neoadjuvant (induction), concurrent, or postoperative adjuvant setting. Studies have revealed that administering chemotherapy concurrently with radiation therapy can improve the therapeutic ratio, resulting in improvement in disease control and survival rates.9,10,11,76
Before a patient receives radiation therapy, the target volume and the surrounding unaffected structures must be defined. The target volume can consist of gross tumor and potential sites of microscopic extension, as well as a volume of tissue to allow for variations in daily patient setup. These volumes are determined by the treating radiation oncologist through physical examination and diagnostic imaging techniques. Treatment planning starts with a simulation—a procedure that is implemented with the use of a special radiographic unit that can reproduce the geometric conditions of a patient on the radiation therapy machine.36 During simulation, the patient is usually placed in the treatment (i.e., supine) position. Patients are immobilized, usually with a thermoplastic mask, to ensure that the treatment position can be reproduced. Following immobilization, the treatment fields are delineated with the assistance of fluoroscopy and diagnostic radiographs. A CT scan for dosimetry is provided as part of the treatment planning session. The target volume is determined, and normal structures are localized. Treatment portals are designed and verified radiographically. The radiation oncologist prescribes the tumor dose (including fractionation schedules) and, in cooperation with a physicist and a dosimetrist, calculates doses, computes beams, and generates isodose curves.36 Treatment is implemented by the radiation therapy technician under the supervision of the treating physician and in consultation with the physicist. Periodically, localization films are used and doses on the charts are verified. Tumor response and the patient’s tolerance of the radiation treatment are routinely evaluated. The patient is usually evaluated once, weekly by the radiation oncologist.
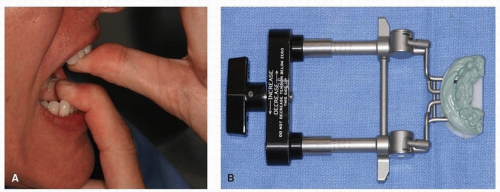 Figure 29.8. A: Physical therapy with active stretching may be helpful for maintaining the oral opening. B: A JDS device will stretch fibrotic tissues during healing and radiation therapy. |
Radiation Stents
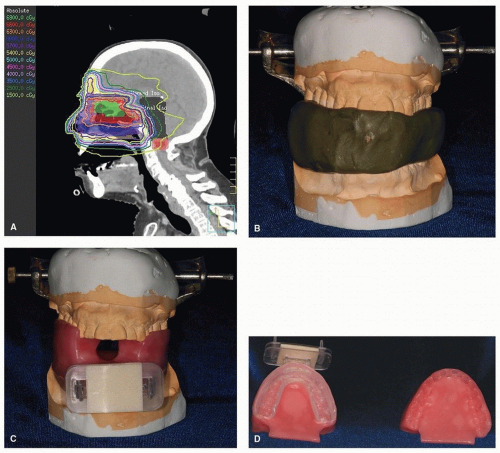
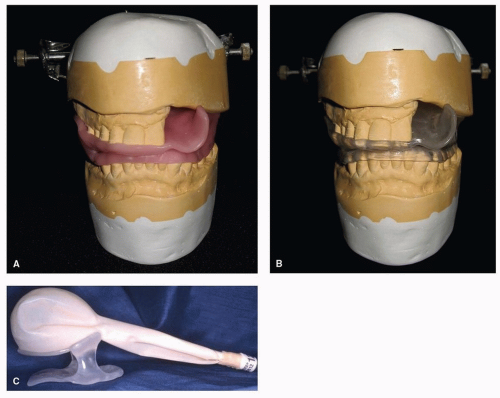
A custom intraoral radiation device, often referred to as a radiation stent, can reduce the radiation dose to unaffected tissue by displacing normal tissue away from the treatment field, minimizing complications and side effects of therapy without compromising total dose to affected area. Patients whose primary tumor is in the oral cavity, oropharynx, paranasal/maxillary sinus, and salivary glands benefit the most by repositioning critical tissue away from the beam of radiation. These radiation stents can be used to depress the mandible and tongue beyond the radiation field, thus minimizing radiation to tissues not at risk or not diseased (e.g., in patients with maxillary sinus cancer, nasopharynx or nasal cancer) (Fig. 29.9A).
These fully customizable devices can be fabricated easily, without complicated prosthodontic techniques. Maxillary and mandibular impressions are required, using a relatively inexpensive material, such as irreversible hydrocolloid. Casts fabricated from these impressions are then placed on a simple hinge articulator with the use of an interocclusal record made at an appropriate mouth-opening distance. The distance is dependent upon the type of device needed, that is, a mouthopening tongue-depressing stent requires 2 cm opening versus a mouth-opening tongue-deviating radiation stent, which requires 5 mm opening. These devices are also customizable based on the type of radiation to be administered. Proton radiation stents require the addition of a customizable “bite block” (Fig. 29.9B). This bite block attaches to the mask, which allows for a repeatable positioning.
In contrast, the tongue-depressing/mouth-opening radiation device can be fabricated to position the oral tongue and mandible into the field of radiation treatment for repeatable positioning of the oral cavity and tongue during external beam
treatment (Fig. 29.10).77 This approach, along with an aquaplast mask, allows for a therapy of exactness and thus eliminates error caused by movement. Such stents are relatively simple to fabricate and are supplied by the oral oncologist.77,78 If oral oncologic support is not readily available, the radiation therapist or the radiation staff can fabricate a simple device with a tongue blade (composed of Lucite) and a large cork held together with tape, although this measure is not strongly advocated.
treatment (Fig. 29.10).77 This approach, along with an aquaplast mask, allows for a therapy of exactness and thus eliminates error caused by movement. Such stents are relatively simple to fabricate and are supplied by the oral oncologist.77,78 If oral oncologic support is not readily available, the radiation therapist or the radiation staff can fabricate a simple device with a tongue blade (composed of Lucite) and a large cork held together with tape, although this measure is not strongly advocated.
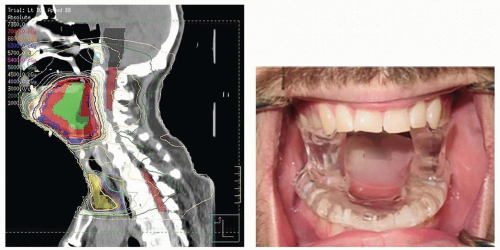
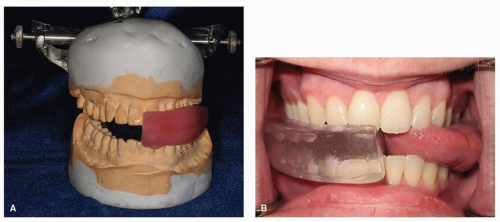
In circumstances requiring radiation of the floor of mouth or anterior oral cavity, the stent design can be modified to protect the oral tongue. Replacing the horizontal tongue blade portion with an inclined ramp and placing the ventral tongue on the ramp permit the tongue to be positioned posterior to the primary treatment field (Fig. 29.11).
A further modification of the basic stent design is required if a maxillectomy, a procedure that results in an oroantral communication, has been performed (Fig. 29.12). Incorporation of a cradle-like modification into the stent design allows a water-filled latex balloon bolus (Faultless Balloon Rubber Co., Ashland, Ohio) to be supported within the surgical defect, thus eliminating the air gap.79 This device, along with placement of a tissue-equivalent material in the defect (i.e., saline), permits a more homogeneous energy distribution.
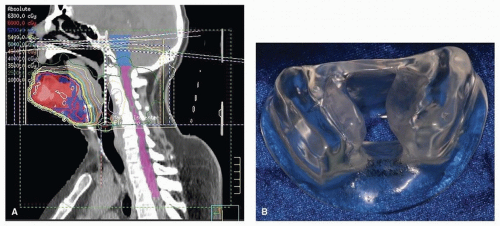
Patients who undergo radiation involving a unilateral treatment field benefit from a unilateral radiation device (Fig. 29.13). The device, known as a mouth-opening tongue-deviating radiation stent, deviates the tongue and associated tissues to the contralateral side, thus reducing treatment-related morbidity to the
contralateral tissues. A Lipowitz alloy is added to the unilateral radiation stent for added protection when the treated tissue volume receives electron beam energy or combined electron/photon energy weighted 4:1.
contralateral tissues. A Lipowitz alloy is added to the unilateral radiation stent for added protection when the treated tissue volume receives electron beam energy or combined electron/photon energy weighted 4:1.
Oral Complications from Radiation Therapy
Good oral health at the onset of radiation therapy minimizes the risk of complications from therapeutic administration of ionizing radiation to the head and neck. These consequences can be categorized as either acute (e.g., mucositis, infectious stomatitis, alteration of taste or smell acuity, dermatitis, pain, inflammation, and difficulty swallowing) or chronic (e.g., xerostomia, caries, abnormal development, fibrosis, trismus, photosensitivity, ORN, and pain).1,37,45 The severity of treatment-induced morbidity depends on multiple factors, including radiation dose, energy source, volume of tissue treated, pretreatment performance status, and pretreatment periodontal condition.55 The radiated tissue is susceptible to dermatitis and mucositis, which are often accompanied by salivary gland hypofunction, dysgeusia, dysphagia, odynophagia, hypovascularity of soft and hard tissues, fibrosis, or trismus.1,22,54,55 Widespread oral
melanotic hyperpigmentation and hypopigmentation have been reported. Developmental abnormalities of the dentition and jaws may occur in children undergoing head and neck radiation therapy.80,81,82 In patients of all ages, altered tissues within the volume of tissue radiated are highly susceptible to infectious processes, with fungal organisms such as Candida albicans or other Candida species; bacterial infections, including streptococci and staphylococci; and viral infections, especially HSV.59
melanotic hyperpigmentation and hypopigmentation have been reported. Developmental abnormalities of the dentition and jaws may occur in children undergoing head and neck radiation therapy.80,81,82 In patients of all ages, altered tissues within the volume of tissue radiated are highly susceptible to infectious processes, with fungal organisms such as Candida albicans or other Candida species; bacterial infections, including streptococci and staphylococci; and viral infections, especially HSV.59
Radiation-Induced Mucositis
Oral mucositis generally occurs 5 to 7 days after initiation of external beam radiation therapy. Oral mucosal changes depend on fractionation, energy source, total dose of radiation, and oral and dental status (Fig. 29.14).55 During conventional daily dosing, the rate of destruction of the basal cell layer in excess of proliferation of new cells creates a net deficit of cells resulting in mucositis. During the course of radiation therapy, the mucosa becomes thin as a result of direct cell death and the sloughing off of epithelial cells.36,83 Subepithelial edema can evoke an epithelial breakdown. By the end of treatment, diffuse erythema, ulceration, spontaneous bleeding, and white or yellow pseudomembrane formation may be present.84 Late, radiation-induced changes in the oral mucosa are thought to be due primarily to damage of the microvasculature and the connective tissue stromal elements. Telangiectasia, occlusion of capillaries, thickening of blood vessel walls (lack of tonus), and increased hyaline and collagen deposition contribute to thin, atrophic, and relatively avascular mucous membranes.76,83 Such changes predispose the patient to hypersensitivity to trauma, infection of the oral mucosa, and delayed wound healing, particularly after minor surgical procedures.1 Dental hygiene procedures may lead to an increased risk of infection or chronic ulceration during this period.4,23,37
The grade of oral mucositis can be partially controlled by the elimination of all secondary sources of irritation, such as alcohol, smoking, plaque and calculus, coarse or hot foods, alcohol-or phenol-containing mouth rinses, and sodium products that can further dehydrate oral tissues.21,84 Many physicians recommend that mouth rinses containing alcohol, phenolics, or astringents be avoided owing to the potential of such agents to dehydrate the mucosa and increase oral discomfort.1,45,55,82 Treatment of mucositis typically consists of palliative pain reduction therapy; however, the Multinational Association of Supportive Care in Cancer (MASCC), in partnership with the International Society of Oral Oncology (ISOO), completed a review of the relevant literature of treatments for mucositis in 2013, which identified low-level laser therapy (LLLT) as the most promising treatment.85,86,87,88,89 A new suggestion was made by this group for LLLT (wavelength approximately 632.8 nm) for the prevention of oral mucositis for patients undergoing definitive radiation therapy without concomitant chemotherapy.86 However, this strategy has yet to be widely practiced in major centers and remains investigational.
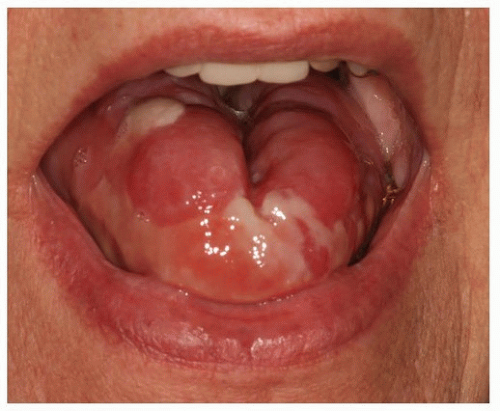 Figure 29.14. Radiation-induced mucositis, which has been exacerbated by use of an alcohol-containing mouth rinse. |
Gingival tissues are sensitive to radiation therapy, and increased gingival recession may occur without signs or symptoms of periodontal inflammation.23 This recession may be due to the hypovascularity induced by the radiation and to the marked reduction of the quality and quantity of salivary secretions following irradiation.90,91 There is evidence that a limited amount of gingival revascularization may occur over time, provided that the total radiation dosage delivered was relatively low.35
Superinfection
Good oral hygiene is essential to improving oral comfort and reducing the risk of oral contamination. Bacterial, fungal, and viral infections can occur as superinfections with mucositis but are less likely to induce septicemia in patients undergoing radiation therapy than in patients receiving chemotherapy.1 Patients receiving a concurrent regimen of radiation therapy and chemotherapy may be at greater risk of infectious mucositis than patients treated with either therapy alone, due to increased relative dose intensity and immunosuppression secondary to the myelosuppressive effects of chemotherapy.
With fungal infections, such as oral candidiasis, the pathogens can be invasive, refractory to treatment, and potentially systemically disseminated.59,82 Candidiasis can manifest as pseudomembranous, hyperplastic, or atrophic (erythematous) oral lesions.25,59 The sites most frequently affected are the tongue, buccal mucosa, hard or soft palate, and commissura labiorum oris (i.e., angular cheilitis). Diagnosis of candidiasis is based on an exclusion of therapy-related toxicity and a clinical evaluation that includes fungal cultures or visualization of organisms using potassium hydroxide stain or Gram stain.1,92,93 After the diagnosis has been confirmed, treatment consists of topical antifungal agents such as nystatin oral suspension or clotrimazole troches, depending on the degree of xerostomia.27,53,59 Patients with unresponsive or disseminated candidiasis may require systemic therapy, such as with ketoconazole, fluconazole, itraconazole, amphotericin B (liposomal), or a combination of antimicrobials with antifungal therapy ideally under the care of a specialist in infectious disease.27,59
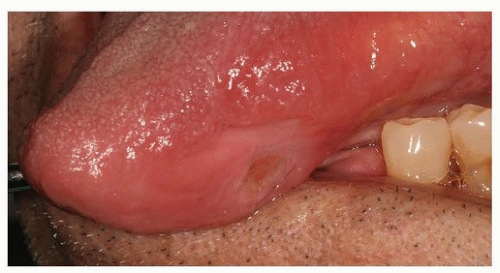
At times, infections may manifest in the several weeks following the completion of radiation, during the healing phase (Fig. 29.15). These types of lesions typically present as a nonhealing ulcers generally within the field of radiation and can be confused easily with mucositis. Long-standing lesions must be cultured and sensitivity tests completed to establish a causative infective agent. Following identification, the appropriate antibiotic, antifungal, or antiviral should be prescribed.
Osteoradionecrosis
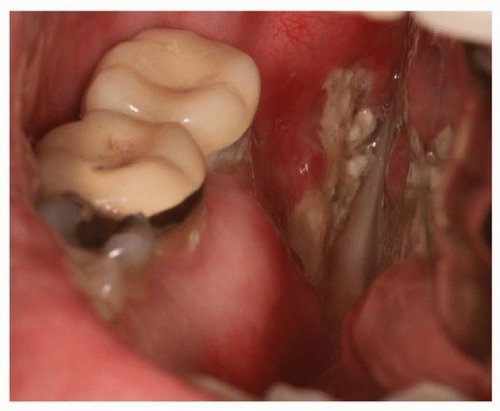
Radiation can permanently destroy cellular elements as well as the vascularity of bone and thus limit the potential for wound maintenance and the ability to heal after infection or trauma (e.g., dental extraction, alveoloplasty).94,95 Further, the risk of complications following trauma or oral surgical procedures in an irradiated field can be highly significant, depending on a predetermined threshold of radiation, and can result in ORN (Fig. 29.16).54,55,57,95 For these reasons, elective oral surgical procedures, such as soft tissue surgery, are contraindicated within the radiated field owing to the potential for treatment-induced hypovascularity, hypocellularity, and hypoxia; however, noninvasive dental procedures can safely be performed including oral prophylaxis, radiography, and direct restorative (i.e., fillings), endodontic, and prosthodontic procedures (i.e., crowns and dentures).1,37,45 Optimal oral health must be maintained during and after radiation therapy; however, to avoid soft tissue injury during the postradiation healing period, patients must curtail all but the most basic oral hygiene procedures (i.e., tooth brushing, flossing, and fluoride therapy). It is important after radiation therapy that dental caries or traumatic injury that could lead to ORN be detected and treated; however, ORN can occur spontaneously.96,97
If oral or periodontal surgical intervention is required after radiation therapy, the clinician should discuss with the treating radiation therapist the volume of tissue radiated and specific treatment parameters and should request a copy of the treatment summary and the dosimetries. Generally, oral surgery, that is, dental extraction or endosseous implant placement, is discouraged in tissue that has received high-dose radiation due to risk of ORN and increased implant failure. Similarly, implants placed into radiated osteocutaneous fibula reconstructions have also shown an increased rate of failure.98 For teeth that are nonrestorable, conservative measures such as endodontics and crown amputation are preferred over dental extraction. In cases where oral surgery is unavoidable, preoperative hyperbaric oxygen (HBO) therapy administered after radiation therapy is thought to increase the potential for wound healing while reducing the risk of ORN by promoting angiogenesis and osteogenesis, although this remains to be somewhat controversial in the literature.73,99,100 To date, there are no well-structured, randomized clinical trials to suggest that benefits of HBO therapy. There are contraindications to treatment with HBO, including, but not limited to, pneumothorax and distant metastatic presentation; however, decision to use HBO remains based on empirical judgment and clinician dependent.
HBO therapy can be used as an adjunct to debridement (sequestrectomy) in the treatment of ORN, along with parenteral antibiotics (as dictated by bone culture results).99,100 Benefits of HBO therapy include improved wound healing in infected ischemic tissue, osteoclastic stimulation, the restoration of normal defense mechanisms responsible for bacterial killing, and the direct killing of anaerobes. Oral oncologists usually prescribe according to the Marx protocol, consisting of 20 preoperative HBO treatments followed by 10 postoperative treatments.73,101 This prescription may be altered by increasing the number of postoperative HBO treatments to 20 treatments, as decided by the treatment team, to maximize the wound-healing capacity of the patient; however, little benefit was found in patients who received over 40 total treatments. Along with HBO therapy, a specific oral care regimen is indicated to augment wound healing. In such cases, tissues should be managed gently, that is, flapless dental surgery, and antibiotic coverage is required. Local anesthetics containing epinephrine should be avoided, when possible, to prevent further vascular constriction.21 Successful placement of endosseous implants in radiated fields with a pretreatment regimen of HBO therapy has been reported; however, there are also reports of initiation of ORN by such elective surgical intervention even when appropriate measures were taken.96,97
HBO therapy is time-consuming and expensive and must be performed in an accredited wound care center. When compared with postradiation oral surgical treatment consisting of radical debridement and reconstruction, HBO treatment can be cost-effective if extensive surgery is avoided. HBO may preclude the need for jaw resection and microvascular surgery. In cases in which resection and reconstruction are inevitable, there seems to be minimal benefits from preoperative HBO.
Dental Caries
Dental caries is a common postradiation morbid sequela that is often exacerbated by xerostomia (see Fig. 29.17A and B). Radiation of major salivary glands leads to qualitative and
quantitative changes in salivary secretions,1,102,103 thus increasing plaque and mucoid debris accumulation and reducing the salivary pH as a result of a decreased buffering capacity of saliva.91 These conditions create a cariogenic oral environment, particularly in patients ingesting a diet high in carbohydrates or sucrose.
quantitative changes in salivary secretions,1,102,103 thus increasing plaque and mucoid debris accumulation and reducing the salivary pH as a result of a decreased buffering capacity of saliva.91 These conditions create a cariogenic oral environment, particularly in patients ingesting a diet high in carbohydrates or sucrose.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree