Total mesorectal excision (TME) remains the gold standard for rectal cancer because it provides superior oncologic outcomes compared with local excision (LE). LE can be offered as an alternative for carefully selected patients; however, it must be emphasized that even in ideal patients, LE does not achieve equivalent results regarding oncologic outcomes compared with TME. With LE, patients trade a higher cancer cure rate for a lower risk of mortality and lower morbidity. The role of chemoradiation and LE in the treatment of rectal cancer is still under study.
The ultimate goal of the treatment of rectal cancer is to cure the disease while preserving function and quality of life. Total mesorectal excision (TME), the surgical removal of the rectum and its mesorectal envelope, is the accepted standard approach for the treatment of rectal cancer. Patients with tumors located in the middle or upper rectum often undergo an anterior or low anterior resection, preserving the anal sphincter, whereas patients with distal tumors require a complete abdominoperineal resection of the rectum, resulting in permanent colostomy. Patients with early-stage (stage I) rectal cancer who undergo this aggressive surgical approach benefit from a high cure rate, with 5-year survival rates reported between 87% to 90%. TME, however, is a major operation that is accompanied by significant mortality (1%–6%) and considerable morbidity. Anastomotic leakage is reported in 5% to 15% of patients undergoing low rectal anastomosis, and genitourinary dysfunction occurs in up to 30% to 40% of patients. Functional disturbances, including bowel urgency, tenesmus, soiling, and fecal incontinence, are also commonly reported, as is depression, developing in 10% to 32% of patients. These sequelae often persist and have a significant impact on quality of life. Nonetheless, the associated morbidity and mortality of this surgical intervention are currently justified by the oncologic control provided by this approach.
Local excision (LE) has always been an accepted alternative in patients unfit for radical surgery because of advanced age or comorbid conditions. More recently, LE has been explored as an alternative to TME in selected patients with early-stage rectal cancer. It is appealing because it is less invasive, alleviates the need for a colostomy or the distressing sequelae related to low colorectal anastomosis, and results in low morbidity and mortality. But LE alone results in higher rates of local recurrence that, albeit occasionally salvageable by TME, could ultimately compromise long-term survival. Consequently, LE as a curative surgical approach for early rectal cancer has been treated with caution and has yet to gain widespread acceptance. The conventional transanal excision (TAE) is the most widely used approach for LE, but transanal endoscopic microsurgery (TEM) is gaining popularity as a possible alternative, particularly for tumors of the upper rectum that cannot be reached by standard TAE.
The oncologic benefits of neoadjuvant chemoradiation (CRT) observed in patients with locally advanced rectal cancer treated with TME has hastened interest in investigating whether or not CRT could play a role in reducing recurrence after LE in patients with early rectal cancer. Several recent studies suggest that if chemotherapy and radiation therapy are given before LE, the risk of recurrence drops to a level comparable with TME. Although still controversial, LE in combination with preoperative CRT may, therefore, have an expanding role in the treatment of early-stage rectal cancer.
The expansion of colorectal cancer screening programs is increasing the proportion of patients diagnosed with early rectal cancer. The aging of the population is increasing the proportion of high-risk elderly patients with early-stage rectal cancers who may benefit from a less-morbid surgical treatment. These changes probably explain why, in spite of the paucity of information about the advantages of LE compared with TME, the proportion of patients having LE has increased in recent years. The aim of this review is to analyze the current literature to try to identify rectal cancer patients who may benefit from LE.
Patient selection criteria for local excision
The ideal candidates for LE, either by TAE or TEM, are patients with rectal cancers localized to the bowel wall, meaning tumors that do not penetrate beyond the muscularis propria and have not metastasized to the perirectal nodes. Because most of the mesorectum is left relatively undisturbed during LE, any tumor cells left in the perirectal fat or the mesorectal lymph nodes may lead to local recurrence and compromised survival. Unfortunately, preoperative clinical findings and imaging studies are not completely accurate in assessing the depth of tumor invasion of the rectal wall and the status of the mesorectal lymph nodes, and patient selection remains one of the most important barriers to the adoption of LE.
Clinical Preoperative Evaluation
Although tumor stage remains the most important criteria for patient selection for LE, several gross features, such as tumor mobility, size, morphology, and distance from the anal verge, should also be taken into consideration. Digital rectal examination and proctoscopy provide surgeons with useful information about the tumor and the patient, which is essential for the treatment decision making. Patients with large and fixed tumors can be immediately excluded from consideration for LE. Although tumor size is not a good predictor of depth of invasion or nodal metastasis, patients with tumors larger than 4 cm in diameter or involving more than 40% of the circumference of the rectum are poor candidates for LE. The mobility of a tumor on digital rectal examination also provides clues about its depth of invasion. Distinguishing tumor invasion within individual layers of the rectal wall, however, is beyond the capabilities of a clinical examination. Patients with tethered or fixed tumors, which probably invade into the perirectal fat or surrounding tissue, are poor candidates for LE. Tumor morphology and distance from the anal verge do not have independent prognostic value when stratified by tumor stage. Distance from the anal verge, however, is important for patient selection. LE is favored in patients with lower lesions because there is a higher risk for permanent colostomy or poor functional results if they are treated with restorative TME.
Radiologic Preoperative Staging
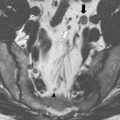
Techniques, such as CT scan and surface coil MRI, are useful for evaluating the abdomen for distant spread and assessing locally advanced tumors. The new-generation CT and MRI are able to detect tumor invasion outside the bowel wall and predict the relationship of the tumor with the circumferential margins ; however, they do not provide sufficient resolution of the layers of bowel wall to distinguish between a T1 and a T2 lesion or between a polyp and a T1 lesion. Only endorectal ultrasound (ERUS) and endorectal coil MRI can provide this level of detail. Either of these studies should, therefore, be performed in the evaluation of any patients considered for LE.
In a systematic review of 53 studies, including 2915 patients, Kwok and colleagues reported the accuracy of ERUS to be 87% for T-stage and 74% for lymph node involvement. For MRI with endorectal coil, the corresponding figures were 84% and 82%. In a large meta-analysis with data from 90 publications, Bipat and colleagues found the sensitivity of ERUS and MRI for tumor invasion outside the rectal wall as high as 90% and 82%, respectively, but sensitivity for lymph node metastasis was significantly lower at 67% and 66%, respectively.
The largest single-institution case review of 545 patients suggested that the accuracy of ERUS in determining depth of invasion is significantly lower than previously published. Garcia-Aguilar and colleagues cited an overall ERUS accuracy rate of only 69% (with 18% overstaged and 13% understaged) when compared with pathologic staging. ERUS accuracy tended to increase with advancing T stage. The ERUS accuracy for T1 tumors was 47%, 68% for T2 tumors, and 70% for T3 tumors. The accuracy of ERUS for detecting the presence or absence of metastatic tumor deposits in mesorectal lymph nodes was only 64% in this study.
This study illustrates the inherent biases that plague studies evaluating ERUS or any type of imaging technique for rectal cancer. Because the gold standard against which the imaging study is compared is the final unirradiated pathologic specimen, only those patients who did not undergo preoperative radiation are included in the study. The majority of patients with obvious advanced disease on the ERUS undergo neoadjuvant therapy and some patients with clearly localized lesions are offered LE. With exclusion of both of these groups, those who are left for evaluation are patients with more equivocal findings on the imaging studies. This selection bias has led to an underestimation of the accuracy of ERUS and MRI.
Although conventional 2-D ERUS is one of the most adequate modalities for preoperative staging of rectal cancer, its ability to correctly evaluate the depth of submucosal invasion remains controversial and its efficacy with respect to treatment selection has not been clarified in detail. 3-D ERUS is based on the construction of a 3-D model from the synthesis of a high number of parallel transaxial 2-D images. Recent information suggests that 3-D reconstruction increases the accuracy of ERUS in assessing the depth of rectal cancer submucosal invasion and aids in selecting patients for LE.
Histology
The most important predictor of lymph node metastasis is depth of tumor invasion (T stage). Up to 70% of T3/4 tumors and up to 28% of T2 tumors have lymph node metastasis in the mesorectum. An LE with curative intent is not an option for patients with T2 or T3 tumors because of the high risk of leaving cancer cells in the mesorectal lymph nodes. Even for T1 tumors, the risk of nodal metastasis ranges from 10% to 15%, still unacceptably high for LE alone. Attempts to further stratify the risk within this group of tumors have included subdivision of the depth of submucosal invasion and identification of signs of aggressive behavior, such as lymphovascular invasion and poor differentiation. Kudo first introduced the concept of dividing the submucosal layer into three levels (sm1, sm2, and sm3). Sm1 indicates tumor invasion into the upper third of the submucosa, sm2 into the middle third, and sm3 into the lower third. Several studies have demonstrated an association of deeper submucosal invasion with increasing risk of lymph node metastases in 0% to 3% for sm1 lesions to 20% to 23% for sm3 lesions. Kitajima and colleagues also showed on multivariate analysis that submucosal invasion deeper than 1000 μm was associated with a 5-fold increased risk of lymph node spread (odds ratio 5.4).
Several histologic features have also been associated with a more aggressive subset of T1 tumors with a higher propensity for lymphatic and distant spread. Blumberg and colleagues reported that poor differentiation and lymphovascular invasion were associated with rates of lymph node metastases of 30% and 33% respectively. Willett and colleagues published an actuarial 5-year survival rate of only 79% in early rectal cancer (T1 or T2) when associated with these adverse histologic features compared with 91% in the presence of favorable histology. Tumor budding (defined as isolated cancer cells or nests of cancer cells in normal tissue at the edge of the main tumor) in T1 rectal cancers has been associated with lymph node metastases in 25% of cases and has been predictive of worse survival after TME independent of disease stage.
Although the use of histologic criteria is fraught with pitfalls, including sampling error and interobserver variability, the evidence indicates that high-grade and lymphovascular invasion predict a higher risk of lymph node involvement. Other factors, such as depth of submucosal invasion and tumor budding, are less widely used but also suggest an increased risk. The presence of any of these adverse histologic findings after LE should prompt an unequivocal recommendation for further treatment.
In addition to depth of tumor invasion, histologic type, and lymphatic invasion, gender may be a predictive marker for lymph node metastasis in early rectal cancer. Kobayashi and colleagues showed that approximately 1% of men with well-differentiated T1 adenocarcinoma of the lower rectum had lymph node metastasis. Such patients were deemed suitable candidates for LE. In contrast, the rate of lymph node metastasis in women with histologic types other than well-differentiated adenocarcinoma was 30.4%, even when the tumor did not invade the muscularis propria, suggesting that these patients should not undergo LE but rather TME.
Technical aspects
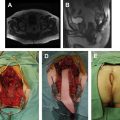
TAE of rectal tumors, first described by Parks and colleagues, requires a full bowel preparation and perioperative antibiotics. The procedure is performed under general or regional anesthesia and the patient is placed in either jack-knife prone or lithotomy position, depending on the location of the tumor. Use of a pudenal block assists with relaxation of the sphincter as well as postoperative analgesia.
A Lone Star retractor (Lone Star Medical Products, Inc, Stafford, TX, USA) is used to efface the anus and facilitate exposure of the distal rectum. A Pratt bivalve speculum and Sawyer, Hill-Ferguson, or Ferguson Moon retractors are also used to dilate the anus and visualize the tumor within the rectum. Deeper lesions often require the use of narrow Deaver or even Wylie renal vein retractors. Use of a headlamp is essential to provide sufficient illumination of the field.
Electrocautery is used to define a 1-cm circumferential margin around the tumor. Traction sutures can be placed in the normal tissue around the tumor for better exposure and manipulation. Excision must encompass the full thickness of the rectal wall with the dissection extending into the surrounding mesorectal fat. Additional sutures can be placed in the proximal edge of the normal rectum to aid in closure of the defect. Once the specimen is fully excised, it should be oriented and pinned before sending it to pathology. The rectal wall defect is closed transversely and a proctoscopic examination is performed at the end of the procedure to assure the rectum is unblocked. TAE is a safe procedure with minimal morbidity. Postoperative pain and discomfort are usually minor. Patients may eat on the evening of the operation, and most are discharged with 48 hours of the operation. Serious complications, including local sepsis, fecal incontinence, rectovaginal fistula, and rectal stricture, are rare.
TEM has been used successfully in the management of rectal adenoma and in selected cases of rectal carcinoma. It offers a minimally invasive alternative to TAE, with endoscopic magnification and illumination that provide excellent visualization and multiple instruments that allow precise resection and secure suture closure. A 40-mm operating rectoscope is placed and CO 2 is insufflated into the rectum to provide exposure. Special instruments are used through the rectoscope to excise the tumor and close the defect in the rectal wall. The removal of the tumor follows the same guidelines as standard TAE. This technique allows excellent visualization and access for more proximal tumors. Distal lesions are more difficult to excise with TEM because the seal between the rectoscope and the anal canal is insecure and this prevents proper insufflation of the rectum. Like the standard transanal approach, TEM is also a safe procedure with a low rate of complications. Although there has been concern about anal sphincter injury from prolonged stretch, several studies have documented no lasting adverse effect on anorectal function.
Given the inaccuracy of the clinical staging of rectal cancer, underestimation of the level of tumor invasion of the rectal wall is common. Consequently, a partial-thickness excision of the bowel wall (either by TAE or TEM) carries a high risk of positive resection margins in understaged tumors. In a recent study of 424 patients with rectal tumors treated by TEM, Bach and colleagues found that a partial-thickness excision of the bowel wall was associated with a 6-fold increase in the risk of an R1 resection compared with full-thickness excision. A partial excision should only be performed for benign lesions provided the surgeon is prepared to perform a full-thickness excision or a TME for unsuspected invasion in the surgical specimen. All other patients should have a full-thickness excision. LE should always be viewed as an excisional biopsy of the tumor, after which the need for any additional therapy can be determined.
Technical aspects
TAE of rectal tumors, first described by Parks and colleagues, requires a full bowel preparation and perioperative antibiotics. The procedure is performed under general or regional anesthesia and the patient is placed in either jack-knife prone or lithotomy position, depending on the location of the tumor. Use of a pudenal block assists with relaxation of the sphincter as well as postoperative analgesia.
A Lone Star retractor (Lone Star Medical Products, Inc, Stafford, TX, USA) is used to efface the anus and facilitate exposure of the distal rectum. A Pratt bivalve speculum and Sawyer, Hill-Ferguson, or Ferguson Moon retractors are also used to dilate the anus and visualize the tumor within the rectum. Deeper lesions often require the use of narrow Deaver or even Wylie renal vein retractors. Use of a headlamp is essential to provide sufficient illumination of the field.
Electrocautery is used to define a 1-cm circumferential margin around the tumor. Traction sutures can be placed in the normal tissue around the tumor for better exposure and manipulation. Excision must encompass the full thickness of the rectal wall with the dissection extending into the surrounding mesorectal fat. Additional sutures can be placed in the proximal edge of the normal rectum to aid in closure of the defect. Once the specimen is fully excised, it should be oriented and pinned before sending it to pathology. The rectal wall defect is closed transversely and a proctoscopic examination is performed at the end of the procedure to assure the rectum is unblocked. TAE is a safe procedure with minimal morbidity. Postoperative pain and discomfort are usually minor. Patients may eat on the evening of the operation, and most are discharged with 48 hours of the operation. Serious complications, including local sepsis, fecal incontinence, rectovaginal fistula, and rectal stricture, are rare.
TEM has been used successfully in the management of rectal adenoma and in selected cases of rectal carcinoma. It offers a minimally invasive alternative to TAE, with endoscopic magnification and illumination that provide excellent visualization and multiple instruments that allow precise resection and secure suture closure. A 40-mm operating rectoscope is placed and CO 2 is insufflated into the rectum to provide exposure. Special instruments are used through the rectoscope to excise the tumor and close the defect in the rectal wall. The removal of the tumor follows the same guidelines as standard TAE. This technique allows excellent visualization and access for more proximal tumors. Distal lesions are more difficult to excise with TEM because the seal between the rectoscope and the anal canal is insecure and this prevents proper insufflation of the rectum. Like the standard transanal approach, TEM is also a safe procedure with a low rate of complications. Although there has been concern about anal sphincter injury from prolonged stretch, several studies have documented no lasting adverse effect on anorectal function.
Given the inaccuracy of the clinical staging of rectal cancer, underestimation of the level of tumor invasion of the rectal wall is common. Consequently, a partial-thickness excision of the bowel wall (either by TAE or TEM) carries a high risk of positive resection margins in understaged tumors. In a recent study of 424 patients with rectal tumors treated by TEM, Bach and colleagues found that a partial-thickness excision of the bowel wall was associated with a 6-fold increase in the risk of an R1 resection compared with full-thickness excision. A partial excision should only be performed for benign lesions provided the surgeon is prepared to perform a full-thickness excision or a TME for unsuspected invasion in the surgical specimen. All other patients should have a full-thickness excision. LE should always be viewed as an excisional biopsy of the tumor, after which the need for any additional therapy can be determined.
Results of TAE
There are many publications reporting the results of TAE alone for the treatment of rectal cancer, but most of them are small, single-institution case series. The reported local recurrence rates range from 0 to 28% for T1 lesions and 11% to 45% for T2 lesions, whereas 5-year survival rates range from 74% to 90% for T1 lesions and 55% to 75% for T2 lesions. The wide variation in reported outcomes probably reflects differences in patient selection, intent of surgery, and surgical technique (reviewed by Kim and colleagues ).
Several retrospective case series and reviews of population-based registries have compared the outcomes of patients with early rectal cancer treated by TAE and TME ( Table 1 ). Although these studies have intrinsic selection bias with patients who had TAE being older and having tumors closer to the anal verge compared with TME patients who tended to be younger and had larger tumors, they provide useful information regarding the outcomes of patients with early rectal cancer. All of them report higher local recurrence rates and lower overall survival for tumors treated by TAE compared with TME. These differences are larger in patents with T2 tumors, but in most series the differences in outcomes between TAE and TME also reach statistical significance for T1 tumors. In one of the largest series published so far, You and colleagues evaluated a cohort of 35,179 patients from the National Cancer Database treated for stage I rectal cancer between 1989 and 2003 and compared the results of 765 patients treated by LE (601 with T1 tumors and 164 with T2 tumors) with 1359 selected patients treated by standard surgical resection. After adjusting for tumor and patient characteristics, the 5-year local recurrence rate after LE was found to be significantly higher than after TME for both T1 tumors (12.5% vs 6.9%; P = .003) and T2 tumors (22.1% vs 15.1%; P = .01). The 5-year overall survival was not influenced by the type of procedure performed for T1 tumors (77.4% for LE vs 81.7% for TME; P = .09) but was lower after LE compared with TME for T2 tumors (67.6% vs 76.5%; P = .01). In this series, no operative mortality was reported for either type of surgery, but patients treated by TME had a 14.6% complication rate compared with 5.6% for patients treated by TAE. In the data from the Swedish Rectal Cancer Registry, the complication rate for LE was 11.5% for patients treated with LE compared with 35.4% for patients treated by low anterior resection and 20.6% for patients treated by abdominoperineal resection.
| Series | T Stage | TAE | TME | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mort | Comp | LR | OS | N | Mort | Comp | LR | OS | FU | ||||
| Hazard et al (2009) | 1 | 418 | (855 a ) | NR | NR | NR | 71% | 1035 | (3465 b ) | NR | NR | NR | 84% * | 3.9 |
| 2 | 155 | 58% | 2005 | 77% * | ||||||||||
| You et al (2007) | 1 | 601 | 0,5% | 5.6% | 8.2% | 77% | 22 | 1.8% | 14.6% | 4.3% * | 82% | >5 | ||
| 2 | 164 | 12.6% | 68% | 83 | 7.2% * | 77% * | ||||||||
| Folkesson et al (2007) | 1,2 | 256 | (643 a ) | NR | 11.5% | 7.2% | 95% | 1802 | (7891 b ) | NR | 35%–41% | 2.8% | 84%–94% | 2.5 |
| Bentrem et al (2005) | 1 | 151 | NR | NR | 15% | 89% | 168 | NR | NR | 3% | 93% | 4.3 | ||
| Endreseth et al (2005) | 1 | 35 | 2.9% | NR | 12% | 70% | 256 | 2.3% | NR | 6% * | 80% * | 2-8 | ||
| Nascimbeni et al (2004) | 1 | 70 | NR | NR | 6.6% | 72% | 74 | NR | NR | 2.8% | 90% * | 8.1 | ||
| Mellgren et al (2000) | 1 | 69 | NR | NR | 18 | 72% | 30 | NR | NR | 0 | 80% | 4.8 | ||
| 2 | 39 | 47 | 65% | 123 | 6 | 81% | ||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree