Abstract
Over the years, several imaging techniques have been used in the diagnosis and management of testicular cancer. We compartmentalize disease stages into preorchiectomy, stage 1, initial stage 2 and 3 and postchemotherapy stage 2 and 3. We then elaborate on various imaging modalities that are relevant to each of these stages. We also describe evolving imaging tools that have shown promise. We attempt to provide a comprehensive review of these techniques over the spectrum of testicular cancer.
1
Introduction
Testicular cancer (TCa) is the most common malignancy among young men aged 20 to 40 years [ ]. This review discusses imaging methods for diagnosing and treating TCa, emphasizing their importance in making clinical decisions.
2
Preorchiectomy
Scrotal ultrasound (US) is the preferred modality for evaluating testicular masses, with sensitivity and specificity exceeding 90% for TCa diagnosis [ ]. Of note, it was found in a recent study that US significantly underestimated testicular lesions less than 2 cm when compared to pathology specimens and that tumor volume (more than maximum diameter) showed a consistent similarity between US and pathology specimens [ ]. Hypoechoic lesions are considered malignant until proven otherwise. Seminomas are characterized as hypoechoic, homogenous masses, and can present with radiographic calcifications or cystic spaces, while nonseminomatous germ cell tumors (NSGCTs) tend to be heterogeneous. Calcifications are also more commonly present in NSGCTs [ ]. In cases where the results are unclear, scrotal Magnetic Resonance Imaging (MRI) can be employed [ ]. It is worth mentioning that follow-up with regular ultrasound scans is not recommended for isolated testicular microlithiasis in the absence of risk factors such as family history and cryptorchidism [ ].
Recent developments showing promise include Contrast Enhanced Ultrasound (CEUS) and Real-time Sonoelastography (RTSE). On CEUS, presence of vascularity suggests a malignant lesion while its absence suggests a benign lesion [ ]. Similarly, rigid elasticity on RTSE indicates malignancy, while soft elasticity suggests a benign lesion. A study by Aigner et al. [ ] demonstrated 100% sensitivity and 81% specificity for differentiating benign and malignant testicular lesions ( Fig. 1 ) .
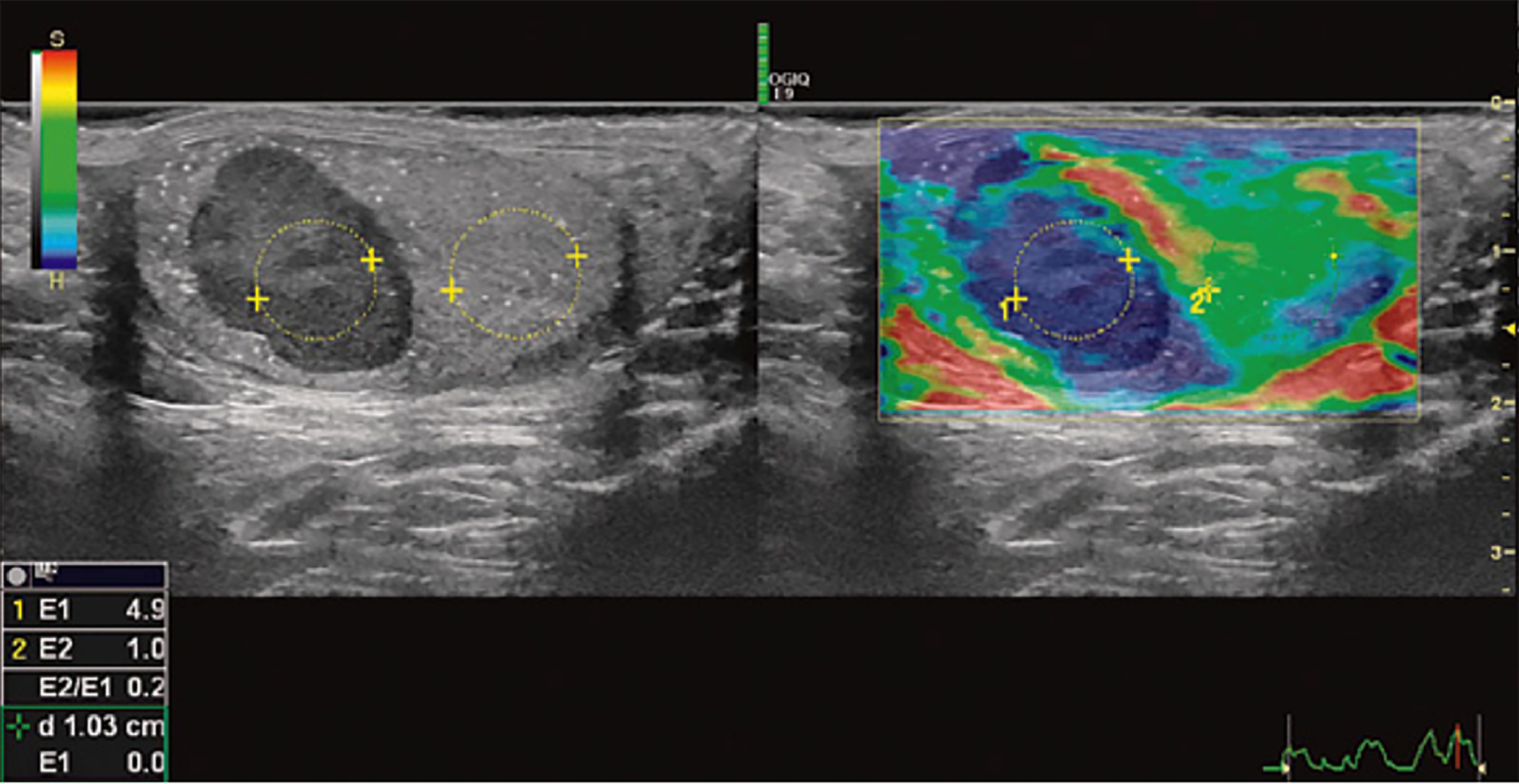
Currently US is the preferred imaging technique initially and for re-evaluation of equivocal masses. Advanced techniques such as MRI, CEUS, and RTSE can provide additional information when US is inconclusive and may have an evolving role in the future.
2.1
Stage 1
For newly diagnosed GCT patients, it is essential for clinicians to acquire a contrast-enhanced abdominal and pelvic computed tomography (CT) or MRI if CT is contraindicated [ ]. For patients with stage 1A or 1B pure seminoma, a chest X-ray (CXR) is the preferred imaging method for assessing for possible lung metastases and nodal disease. If any abnormalities are detected on the CXR, a chest CT with intravenous contrast can be conducted. In contrast, for patients with nonseminoma, stage 2 or 3 disease, or elevated postorchiectomy tumor markers, a chest CT is the preferred imaging modality [ ]. Our preference is to obtain a baseline chest CT at diagnosis, regardless of stage, so that a high-resolution comparator is available should locoregional therapies be required.
According to a study , the relapse rate of NSGCTS was 27.4% within 5 years of diagnosis while that for seminomas was almost half at 15.3% [ ]. Because approximately 90% of relapses occur in the retroperitoneum and a significant number of relapses cannot be detected solely through serum tumor markers (STMs), the primary focus of surveillance is directed towards imaging the retroperitoneum [ ]. Currently, there is no imaging modality that can accurately predict micrometastases; though several efforts have been made, most notably with Positron Emission Tomography (PET) scans.
PET scans initially exhibited exceptional performance characteristics in the postchemotherapy setting for seminoma and may have an evolving role in chemotherapy-naïve and postchemotherapy seminoma management [ ]. However, there is no definitive data that staging PET provided early detection of occult metastases in stage I seminoma.
PET scans have also been investigated in the setting of stage I NSGCT. Huddart et al investigated the utility of Fluorodeoxyglucose (FDG) PET in stage I NSGCTs. In this study, high risk patients with lymphovascular invasion received a PET scan within 8 weeks of orchiectomy. PET positive patients were excluded while PET negative patients underwent surveillance. Within a year, 33 of the 87 PET negative patients relapsed. Therefore, the trial was prematurely terminated due to the high relapse rate of 37.9% in FDG PET/CT negative patients, indicating insufficient negative predictive value for clinical use [ ].
In the case of stage I seminomas and NSGCTs, prospective studies show that PET scans provide limited benefit over CT [ ]. Considering the high cost, radiation exposure, lack of sensitivity for occult metastases, and false positives mandating further testing, the drawbacks of using PET for initial staging outweigh its advantages.
Considerable effort has also been placed on safely reducing the intensity of surveillance imaging in stage I GCT to reduce ionizing radiation exposure, the cost associated with axial imaging, and patient anxiety that may occur around the time of imaging [ ].
A recent study spanning 4 decades revealed that implementation of less rigorous surveillance imaging protocols led to a notable decrease in imaging frequency without compromising patient safety. They included 1583 Clinical stage 1 patients (942 with seminomas and 641 with NSGCTs) and introduced changes in their active surveillance protocols for these patients over a span of 40 years. In the active surveillance protocol from 1981 to 2022, they reduced the total number of CXRs in seminomas from 13 to 1 and CT scans of the abdomen and pelvis from 20 to 10. For NSGCTs, total CXRs were reduced from 27 to zero and CT scans from 11 to 5. They concluded that despite the decrease in number of scans, the relapse rates for both stage 1 seminomas and NSGCTs did not increase [ ].
Similarly, findings reported by the Trial of Imaging and Surveillance in Seminoma Testis (TRISST) highlight the benefits of reducing the frequency of imaging for patients with stage I seminoma and the potential for utilizing MRI as an alternative to CT in these cases [ ]. The rationale for exploring MRI as an alternative to CT is due to an opportunity to decrease radiation. They enrolled 669 patients and randomly assigned them to 4 follow-up arms of 7 CTs, 7 MRIs, 3 CTs and 3 MRIs. These scans were done over a span of 5 years. The primary outcome was the incidence of stage 2C relapse. Relapse occurred in 82 patients (12%), with an equal distribution between the CT and MRI groups. Of note, more relapses were found in the 3-scan group (46) compared to the 7-scan group (36). The investigators thus confirmed the noninferiority of MRIs vs CTs while demonstrating the cost benefits with less frequent imaging schedules.
For patients on active surveillance, the intervals between imaging vary for seminomas and NSGCTs. For seminomas, the relapse rate peaks at 76% within the first 2 years and decreases annually until it becomes 0.3% after the fifth year. Martin et al in their study recommend that the frequency of imaging and visits align with the annual risk of relapse; patients should be imaged every 4 months between years 0 and 2, every 6 months during years 3 to 4, and annually until year 10 [ , ]. The National comprehensive cancer network (NCCN) guidelines recommend CXRs in years 1 to 5 and abdominal/pelvic CT at 3, 6, and 12 months for the first year, every 6 to 12 months for years 2 and 3, and then every 12 to 24 months for years 4 and 5 [ ]. Thus, the consensus is to begin with more frequent imaging for the first 2 years and then gradually taper frequencies until imaging ceases sometime between the fifth to tenth year.
Due to a higher rate of relapse, earlier onset, and a high false negative rate on CT scan, surveillance imaging for NSGCT is more frequent. The NCCN recommends CXRs at 4 and 12 months for the first year and annually from years 2 to 5. Abdominopelvic CT scans are recommended every 4 months for the first year, every 6 months for the second year and then annually till year 5. The frequency increases if risk factors are present [ ].
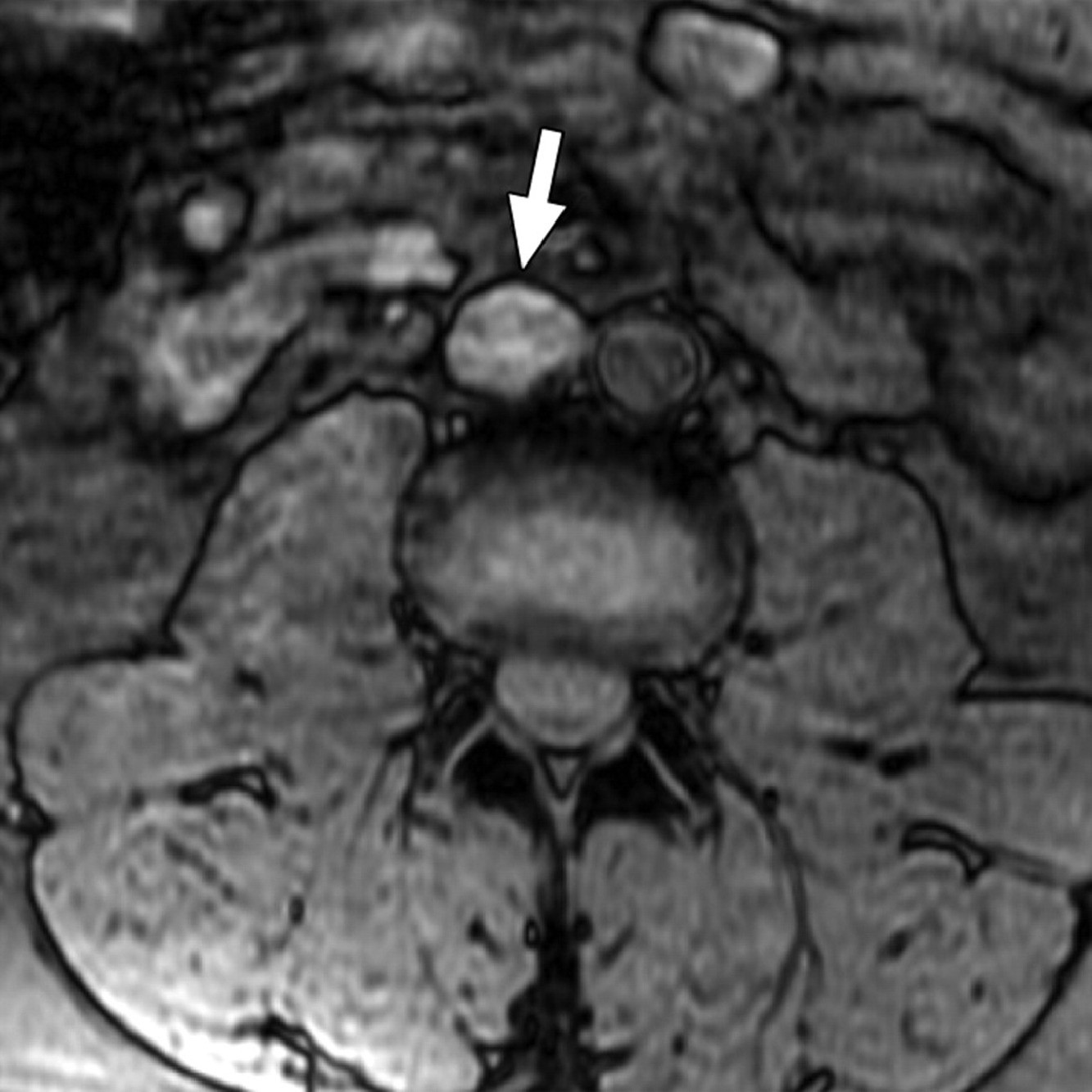
The incorporation of traditional MRI with lymphotropic nanoparticles ( Fig. 2 ) has shown more promising outcomes than conventional CT. Novel MRI techniques enhanced with lymphotropic nanoparticles demonstrated a higher sensitivity (88.2% vs. 70.5%) and specificity (92% vs. 68%) for detecting positive lymph nodes in Stage 1 patients than traditional MRIs. Although these results appear promising, this study was limited by small sample size, lack of confirmatory RPLND (all sentinel node biopsies), and a laborious 2 stage imaging process that occurs over 48 hours [ ]. While promising, further research on advanced imaging techniques is needed for widespread adoption.
In summary, CT scans are preferred for initial imaging after orchiectomy with the evolving role of MRIs, proven to be noninferior to CTs. The imaging schedule can be relaxed after the fifth to tenth year; however, some clinicians prefer to follow their patients indefinitely with CXRs and STMs due to the risk of late relapse of GCTs.
3
Initial staging imaging for Stage 2 and 3 disease
Accurate staging incorporating radiographic and serologic findings dictate care in advanced stage disease. The sensitivity and specificity of CT in the determination of nodal metastases varies depending on the size criteria. In a study using a size threshold of 10 mm or more, CT was 37% sensitive and 100% specific for nodal metastases. Lowering the size threshold to 4 mm or more to indicate involved nodes in tumor landing zones increased the sensitivity to 93% but decreased the specificity to 58% [ ]. Additionally, there is a need to standardize lymph node measurement on cross-sectional imaging of the retroperitoneum, as was highlighted in a German survey study which revealed that 55% Urologists use the short-axis diameter for measuring lymph nodes while 45% use the long-axis diameter [ ].
Notably in low volume stage II NSGCT, up to 30% of patients will have pN0 disease at time of RPLND [ ]. Emerging literature also suggests a similar pN0 rate among stage II seminoma patients receiving RPLND [ ]. Thus, owing to lack of a high-fidelity imaging modality to separate pN0 patients from pN+ patients, it is advisable to repeat short interval imaging (6–8 weeks) with serum tumor markers before arriving to a definitive treatment decision [ , , ]. A lesion stable or progressing in size would indicate a malignant nature while a shrinking lesion is most likely benign. Further, if the patient developed radiographically detectable metastases over a short imaging interval, they may very well not have benefitted from a locoregional intervention. For patients with small volume metastases, definitive treatment should only commence once imaging confirms a lesion that is highly suspicious for metastases.
It is of utmost importance to avoid unnecessary chemotherapy or radiation in patients with equivocal lymph nodes due to long-term toxicities; PET scan can also be misleading [ ]. Currently, no imaging modality reliably differentiate metastases from benign lymph node enlargements.
In conclusion, it is advisable to err on the side of caution before committing to various treatment options for stage 2 patients.
4
Staging of extrathoracic and extraretroperitoneal sites
Brain imaging may be performed if the patient has neurologic symptoms, postorchiectomy serum beta-hCG >5,000 IU/L or AFP >10,000 ng/mL, in case of nonpulmonary visceral metastasis or extensive lung metastases, or if the testicular tumor is a choriocarcinoma. MRI is the preferred modality for brain imaging [ , ].
Dedicated imaging, such as hepatic-protocol MRI for suspicious liver lesions and technetium-99 bone scan or MRI for suspicious bony lesions may also be required.
5
Postchemotherapy imaging
5.1
NSGCTs
In patients with advanced disease who received induction chemotherapy, complete axial restaging including the chest, abdomen, and pelvis is performed generally 4 to 6 weeks after the completion of chemotherapy. Patients with no residual masses on imaging and normal STMs do not require surgery. However, guideline-directed recommendations are to perform RPLND for residual masses >1cm as 50% harbor teratoma, 10% viable GCT elements, and 40% only fibrosis/ necrosis [ , ]. In other words, 40% of patients will have received an “unnecessary” RPLND if they were found to have fibrosis/necrosis only on histopathologic examination. Resultantly, there has been considerable focus on more precise imaging feature and modalities to better select patients who will benefit from RPLND and those that could potentially be safely observed. FDG-PET has been evaluated as a tool to identify residual disease after chemotherapy. In a prospective trial , the false negative rate for NSGCTs was up to 40% [ ]. The current consensus therefore is to not use FDG-PET for NSGCTs after chemotherapy [ , , ].
Radiomics is a growing field that converts medical images into analyzable data, using pattern recognition and advanced analytics techniques to improve diagnostic accuracy. It was showed that a radiomic classifier postchemotherapy could classify lymph node histology with an accuracy of 81% [ ]. A recent study attempted to predict post operative pathology after postchemotherapy RPLND using preoperative CT radiomics for 45 NSGCT patients. On radiomic extraction, first order statistics such as mean, median, 90th percentile and room mean squares had a significant association with positive pathology while other radiomic statistics such as shape and texture analysis were not associated with pathology [ ]. With improvements in data extraction protocols, radiomics could be a potential tool that could help detect persistent disease after chemotherapy.
In summary, CT restaging 4 to 6 weeks following induction chemotherapy guides treatment decisions in NSGCTs. FDG-PET is unreliable with high false negative rates.
5.2
Seminoma
After chemotherapy, an abdominal/pelvic CT scan is recommended at 3- and 6-months following completion of chemotherapy. Residual masses <3 cm can be uniformly observed. Appropriate management for patients with masses >3 cm represents an unmet need.
FDG-PET has been evaluated in this setting. The prospective SEMPET trial initially evaluated the utility of PET scans in this setting, reporting a specificity and sensitivity of 100% and 89% respectively and, a PPV and NPV of 100% and 97% respectively [ ]. A retrospective review validating this trial evaluating the efficacy of PET scans before and after a cut off of 6 weeks since the last chemotherapy cycle also seemed promising. This analysis reported a sensitivity, specificity, negative predictive value, and positive predictive value for viable disease of 82, 90, 95, and 69%, respectively [ ]. However, a recent study reported a low PPV of 23% for PET scans, questioning its value for residual lesions [ ].
Therefore, Albers et al proposed an alternative algorithm for evaluating residual masses after chemotherapy for seminomas that combines serial FDG-PET scanning, CT surveillance, and histological confirmation when needed to guide management decisions and avoid overreliance on a single post-treatment PET scan [ ]. First, they advised delaying the initial surveillance PET imaging until at least 8 weeks after completion of chemotherapy in order to minimize false positives from nonviable persistent inflammation. Patients with a negative PET scan after this interval can undergo routine surveillance as residual masses can generally be safely observed. Patients with a persistently PET avid lesion that is decreasing in size should have a repeat FDG-PET scan at 6 weeks. For masses growing in size or having high standardized uptake values (SUV), biopsy should be performed while a postchemotherapy RPLND should be reserved for patients with a high recurrence risk, unclear histology and ureteral compression. This algorithm attempts to minimize overtreatment from relying on a single post-treatment PET alone.
In summary, FDG-PET remains a controversial tool in the management of advanced seminomas. While its high NPV is helpful in determining if active surveillance is appropriate in PET negative patients, positive results need to be evaluated with care since RPLND or salvage chemotherapy in all positive patients would lead to overtreatment and morbidity. Ultimately, these decisions need to be made at high volume institutions with dedicated multidisciplinary tumor boards.
6
Conclusion
Scrotal US remains the mainstay for initial diagnosis of testicular masses. CT scans and MRI provide further staging information in early-stage disease, with recent studies supporting reduced imaging frequency postorchiectomy to reduce costs without compromising care. Advanced stage patients require CT scans and imaging for extra-abdominal and thoracic metastases, if clinically indicated. Postchemotherapy NSGCT patients should be evaluated with CT scans as PET scans have minimal utility. FDG-PET remains a controversial tool in seminoma patients after chemotherapy, due to high false-positive rates. These patients are often best managed at high-volume centers with a multidisciplinary team.
This article attempts to give readers a comprehensive review of the evolution of imaging modalities across the spectrum of TCa. We’ve also highlighted evolving modalities such as CEUS, RTSE, lymphotropic MRIs and radiomics that have the potential to advance diagnosis and management in TCa patients.
CRediT authorship contribution statement
Kshitij Pandit: Writing – original draft. Dhruv Puri: Writing – review & editing. Kit Yuen: Writing – review & editing. Nuphat Yodkhunnatham: Writing – review & editing. Margaret Meagher: Supervision. Aditya Bagrodia: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
No funding was received for this work.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Appendix
Supplementary materials
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree