Introduction
Hepatitis C virus (HCV) has a significant impact on the healthcare system in the United States and the Centers for Disease Control and Prevention (CDC) estimates that the number of deaths associated with HCV has surpassed the combined deaths from 60 other nationally notifiable infectious conditions, including HIV (human immunodeficiency virus) and tuberculosis [ ]. The last 5 years however have seen a dramatic change in the HCV treatment paradigm, and with the advent of highly effective direct-acting antivirals (DAA) for HCV, we can for the first time envision the eradication of the HCV epidemic.
The rise in death and overdoses related to opioid prescription drug use observed in the United States led to national efforts on restricting the access to prescription opioids, such as opioid prescription guidelines, dose-limit laws, and prescription monitoring programs [ , ]. While these efforts began to take effect, and prescription opioids become harder to obtain, many people with opioid use disorder began to use and inject illicit heroin [ ], a less expensive and accessible substitute for prescription opioids. Coinciding with this national increase in injection drug use (IDU), the number of incident HCV infections has been escalating as IDU represents the most common route of transmission [ ]. The HIV and HCV outbreak in 2015 in Scott’s county Indiana was a wake-up call for the nation. There were 185 HIV infections and 171 HCV infections in a period of 4 years in a county with a total population of 24,000 [ ]. Similar concerning increases in HCV infection have been seen in West Virginia, Kentucky, and Tennessee [ ]. HCV is predominantly concentrated in people who inject drugs (PWID), which is defined as people who report injecting an illicit drug at least once in their life. It should be noted that PWID is a broad definition that includes both people who currently inject drugs (e.g., past month, past year) and former PWID who have permanently ceased injecting drugs [ ].
Epidemiology
Multiple studies have estimated the prevalence of chronic HCV at or close to 1% among the overall US population [ ]. Up until 2005, in the United States, HCV primarily affected the birth cohort between years 1945–1965, also known as the baby boomer generation. This cohort had been identified as the population with the highest prevalence of HCV infection in the United States (3.3%) and was estimated to account for 75% of all HCV infections in the United States [ ]. More recently, however, there has been an alarming increase in HCV infection among people in a younger age group and this is largely related to the opioid epidemic that is sweeping the nation [ ]. For example, recent state surveillance data from 15 US states have shown equal or higher rates of HCV infection among young adults aged 20–39 years old as compared with baby boomers [ ]. On a global scale, estimates of PWID with chronic HCV vary widely, and have relied on rates of HCV antibody positivity, extrapolating that 65%–75% of antibody-positive individuals will be viremic, with chronic HCV. A review in 2011 estimated about 10.0 million PWID may be anti-HCV positive. In the same review, estimates of PWID with hepatitis B infection (HBcAb positive) and chronic hepatitis B (HBsAg positive) were 6.4 million and 1.2 million, respectively [ ]. In another systematic review, investigators estimated 6.1 million PWID living with HCV worldwide. There were wide regional variations, with the United States, Russia, Brazil, and China accounting for 51% of all infections [ ]. Despite the high rates of HCV among PWID, most PWID have never been offered HCV care or treatment [ ].
Natural history of HCV and mortality
Although acute HCV infection is typically asymptomatic, 15%–30% of individuals may develop acute hepatitis within 5–12 weeks of exposure. It is estimated that 65%–75% of persons who get infected with HCV will develop chronic infection, and of the remaining 25%–35% the majority will clear the infection by 6–12 months. Once chronically infected, the rate of progression from cirrhosis is extremely variable and infected individuals may not develop significant sequelae for decades after initial infection [ ]. In patients with chronic HCV, serious liver disease such as cirrhosis develops in 20%–30% of patients in the third and fourth decades after initial infection. In a meta-analysis of PWID with chronic HCV, the estimated time to cirrhosis was 34 years [ ].
Cirrhosis itself can lead to a wide spectrum of liver disease, but once it ensues, patients with cirrhosis have an approximately 5% annual risk of developing decompensated liver disease, which heralds a poor prognosis, repeat hospitalizations, and in the absence of HCV treatment or a liver transplant, progressive deterioration, and death [ ]. In a National Health Institutes-sponsored observational study, 220 patients with HCV-related cirrhosis were followed for approximately 8 years. A primary outcome of death, hepatic decompensation, and hepatocellular carcinoma (HCC) occurred at a rate of 7.5% per year and patients with a Child Turcotte Pugh score of >7 experienced a death rate of 10% per year [ ].
Mortality among PWID is significantly higher than that of the general population [ ]. Causes of death are often associated with injecting drug use, including fatal overdoses and blood-borne infections. PWID are also at higher risk of dying prematurely than the general population [ , ]. Investigators used data from the Global Burden of Disease 2013 to measure the morbidity and mortality among PWID attributable to HBV, HCV, and HIV, including cirrhosis and HCC. They calculated estimates of burden of disease through years of life lost, years of life lived with disability, deaths, and disability-adjusted life-years (DALYs). Globally, in 2013, more than 10 million DALYs were estimated to be attributable to previous exposure to HIV, HBV, and HCV via IDU [ ]. In the United States, HCV causes greater than 18,000 deaths annually [ ], and is the leading cause in the United States of both end-stage liver disease and HCC, and is one of the most common indications for liver transplantation [ , ]. Without imminent action, mortality from HCV is projected to triple over the next decade [ , ]; HCV-related deaths have now surpassed deaths from HIV infection [ ]. Because mortality in PWID with HCV is 12 times that of the general population, we need innovative interventions to screen, treat, and cure HCV among PWID [ , ].
Screening of HCV among PWID and point-of-care testing
In 2012, the CDC established HCV screening recommendations, including a one-time testing of the birth cohort 1945–1965, as well as other individuals perceived to be at increased risk [ ] ( Table 6.1 ).
| All persons born from 1945 through 1965 |
| Anyone who has ever injected illegal drugs |
| Recipients of blood transfusions or solid organ transplants before July 1992, or clotting factor concentrates made before 1987 |
| Patients who have ever received long-term hemodialysis treatment |
Persons with known exposures to HCV, such as
|
| People living with HIV |
| People with signs or symptoms of liver disease (e.g., abnormal liver enzyme tests) |
| Children born to mothers who have HCV |
As a result of the screening recommendations and highly effective treatment regimens for chronic HCV, screening across large healthcare systems has increased [ ]. Unfortunately, the proportion of HCV infections occurring outside the designated birth cohort is growing rapidly and at a minimum, annual HCV testing is recommended in PWID. In 2020, CDC developed two new recommendations [ ]: (1) HCV screening at least once in a lifetime for all adults aged ≥18 years, except in settings where the prevalence of HCV infection is <0.1% and (2) HCV screening for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection is <0.1%.
The screening algorithm for HCV testing begins with an antibody test, which can be conducted using a rapid antibody test (e.g., OraQuick Rapid Antibody Test) or a laboratory-based assay (e.g., enzyme immunoassay, chemiluminescence immunoassay). This initial test provides a result in terms of reactive or nonreactive. A nonreactive result suggests no evidence of HCV antibody, and, consequently, no further action is required. A reactive result should be taken as evidence of presumptive HCV infection and must be followed by HCV RNA testing. If HCV RNA is not detected, the reactive antibody represents past but cleared HCV infection. If HCV RNA is detected, the diagnosis of current HCV infection is confirmed. Next steps should include providing appropriate counseling on risk avoidance, and linkage to medical care and treatment [ ]. Guidelines currently recommend conducting reflex testing, which implies that the RNA test is automatically performed on all reactive HCV antibody specimens. This automatic testing facilitates a complete evaluation within one visit, thus expediting subsequent steps in staging and clinical management ( Fig. 6.1 )
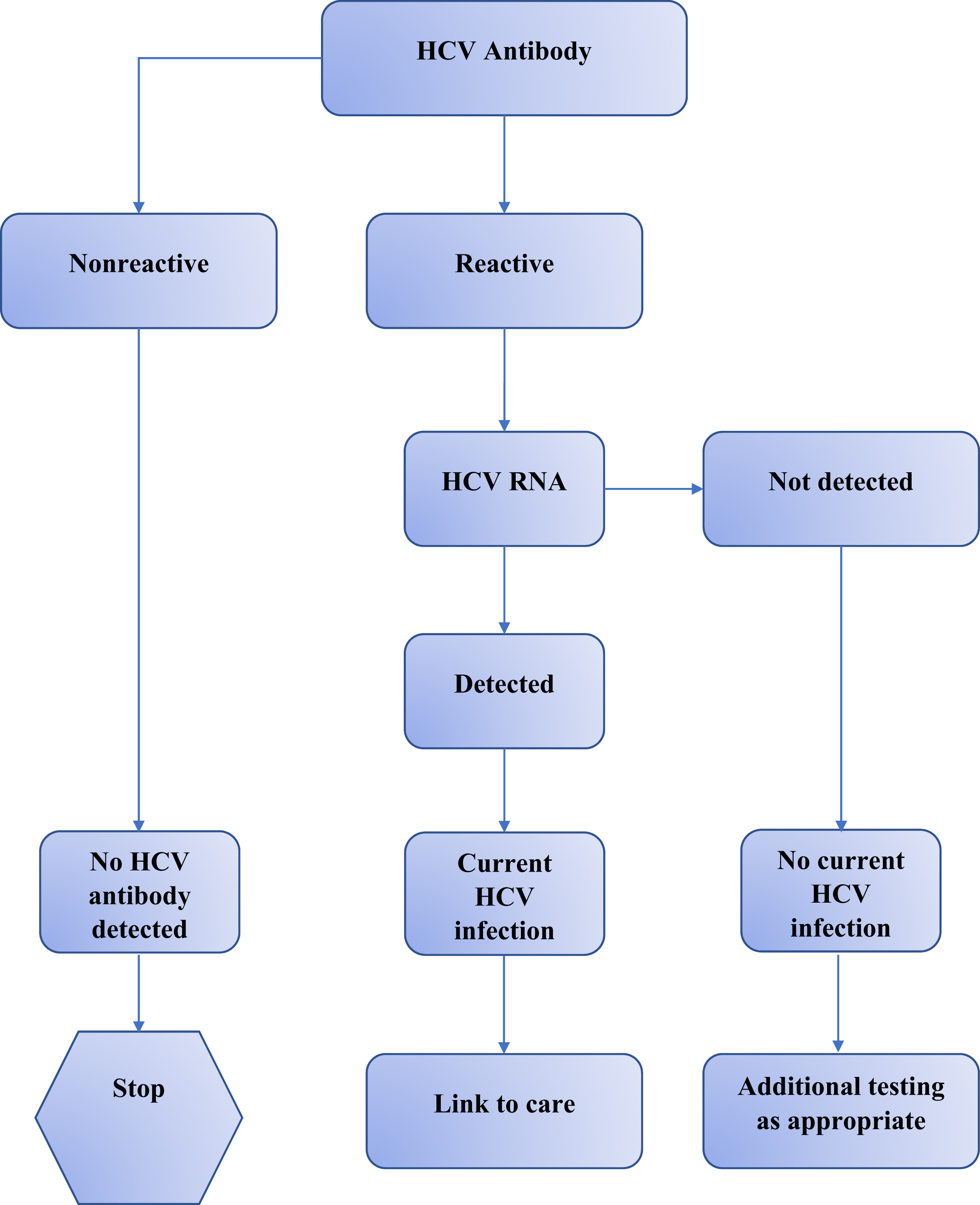
The advent of point-of-care (POC) tests for HCV represents a remarkable advance for HCV diagnosis that have important implications in HCV care. Major advantages of the use of the POC HCV testing are (1) simplification of the testing process; (2) increase in the number of people both tested and given results in the same encounter; and (3) as such may facilitate linkage to care [ ]. POC may be particularly useful for screening vulnerable populations in healthcare, such as PWID. While several POC rapid antibody tests have been developed, only the OraQuick HCV Rapid Antibody Test is approved as POC for HCV testing and CLIA [ ] waived by the Food and Drug Administration. The OraQuick test uses whole blood samples obtained by finger stick or venipuncture, is >98% accurate, and provides results regarding the presence of HCV antibody, either reactive or nonreactive, within 20 min.
Staging of liver disease and HCC screening
HCV-induced hepatic fibrosis is a dynamic process in which chronic inflammation stimulates production and deposition of collagen and extracellular matrix proteins. Assessment of the stage of fibrosis provides prognostic information as well as guidance on the choice and duration of the treatment regimen. The most widely used staging method is the Metavir scoring system which is used to assess the extent of inflammation and fibrosis by histopathological evaluation in a liver biopsy of patients with hepatitis C. Metavir is scored from F0 to F4, where F0 = no fibrosis and F4 = compensated cirrhosis. Liver biopsy was once considered the gold standard for assessment of liver stiffness, but is a painful, invasive, and expensive procedure with a small but not negligible risk of medical complications. Liver biopsies are rarely required anymore for staging and evaluating patients with chronic HCV [ ].
The AASLD/IDSA guidelines currently recommend the use of noninvasive techniques to determine liver stiffness [ , ]. Fibrosis/liver stiffness can be assessed with noninvasive blood tests including Fibrosure/Fibrotest, AST to platelet ratio index (APRI), and FIB-4, among others. Ultrasound-based Transient elastography (TE), more commonly known by the brand name of Fibroscan (Ecosens, France), is a fast (<5 min), noninvasive, painless technique that can be performed in an outpatient clinic [ ] and its results are available immediately. It assesses liver stiffness (fibrosis) by measuring the velocity of a shear wave as it travels through the liver. The higher the velocity, the more advanced the fibrosis. It examines an area of liver tissue 100 times larger than a liver biopsy, and results are expressed in Kilo Pascal (kPa) units. Cut-off values differ between different etiologies of hepatitis (hepatitis B, Alcohol, NASH, etc.) and in HCV values > 9.5 kPa and >12.5 kPa are considered indicative of severe fibrosis (Metavir F3) and cirrhosis (Metavir F4), respectively. The AASLD/IDSA guidelines recommend that both Metavir F3 and Metavir F4 are treated and followed as compensated cirrhosis. Various factors may produce unreliable TE measures and these include obesity (body mass index ≥30 kg/m 2 ), steatosis, cardiac failure, high necroinflammatory activity, ascites, cholestasis, and a nonfasting state.
In general, the noninvasive staging modalities have sensitivities and specificities that range between 75% and 85%, and the combination of liver elastography and a noninvasive blood test has the best ROC (Receiver Operating Curve) for assessing liver fibrosis [ , ].
Risk factors for HCC include cirrhosis of any etiology, and patients with cirrhosis should undergo lifelong surveillance for HCC, because surveillance improves survival and increases the detection of early-stage HCC [ ]. HCV-induced HCC is seen in about 1% of infected individuals after 30 years of chronic infection; however, the risk is significantly higher in cirrhotic patients and is estimated to be 3.5% per year [ ]. In a Veteran Affairs cohort of 3271 persons living with HCV cases of HCC, HCC was highest in patients with cirrhosis together with HCV treatment failure, regardless of whether the treatment was interferon or DAA-based treatment. Sustained virologic response (SVR) was associated with a 71% reduction in HCC . [ ] According to the AASLD guidelines, surveillance for HCC should be with a liver ultrasound (with or without an alpha-fetoprotein) every 6–9 months in patients with cirrhosis [ ].
SVR outcomes in PWID
Despite barriers to receiving care, studies indicate that very high SVR rates can be achieved in the PWID population, due to the new DAA regimens, which are highly efficacious and short in duration.
Glecaprevir/pibrentasvir: In a retrospective analysis of pooled data from 7 phase III trials to evaluate the efficacy and safety of 8 or 12 weeks of glecaprevir/pibrentasvir in patients with chronic HCV infection, in persons who used drugs, SVR rates were achieved by 93% (n/N = 91/98) in people who recently used drugs and 97% (n/N = 591/610) in persons with former drug use. Patients considered as having recently used drugs were those who self-reported IDU within 12 months of screening, had a positive urine illicit drug screen result, or both. In addition, treatment adherence and completion rates were ≥96% regardless of drug use status [ ].
Elbasvir/grazoprevir: In a randomized controlled trial of 301 HCV GT1, 4, 6, infected treatment naïve patients on opioid agonist therapy, the SVR rates after 12 weeks of treatment with elbasvir/grazoprevir were >90% [ ]. Importantly, nearly 60% of participants had positive urine illicit drug screens at baseline, and there was no difference in SVR rates of those with or without a positive drug screen. Adherence greater than 95% (>79 doses) was reported by nearly all participants.
Sofosbuvir/velpatasvir: The coformulated formulation of sofosbuvir/velpatasvir was approved in August 2017 for the treatment of all HCV genotypes. In the SIMPLIFY trial, investigators assessed the efficacy of sofosbuvir/velpatasvir for 12 weeks in 103 participants, of whom 100% had injected drugs in the last 3 months, 74% had injected drugs in the past month, and nearly 60% were on opioid agonist therapy (OAT). The majority were GT3 (58%), 35% had GT1 and 8% participants had cirrhosis. The regimen was well tolerated with one serious adverse event (rhabdomyolysis), and SVR rate was 94% with only one reinfection in the follow-up period [ ].
All DAAs: A recent meta-analysis also provides robust evidence that PWID, both on medications for opioid use disorder (MOUD) and actively injecting drugs, can indeed be successfully treated for HCV with SVR similar to non-PWID [ ]. Including data from 2010 to 2018 of all oral DAA regimens, authors compared HCV outcomes (adherence, discontinuation, SVR) of controls (non-PWID) to over 1700 patients on MOUD and over 500 patients actively injecting drugs. The overall intention to treat SVR was 90% for PWID on MOUD and 88% for those PWID actively using drugs. Importantly, the majority of those who did not achieve SVR were those lost to follow-up (LTFU) rather than true virologic failures, with 43% of those LTFU having completed the full HCV treatment regimen.
Barriers to care for PWID
Despite the evidence that supports treating PWID with HCV DAAs, there is a huge gap in the care continuum and overall <10% of PWID have been initiated these life-saving treatment regimens [ , ]. Innovative methods to get more PWID on HCV treatment and achieve SVR are desperately needed. HCV treatment uptake among PWID has been limited due to multiple interrelated barriers at the level of the patient, the provider, and the health system [ ]. Patient-level barriers include asymptomatic infection and lack of awareness related to HCV and its treatment, which often results in a low perceived need for engagement in care. These issues are compounded by general barriers to healthcare access (e.g., having insurance, a primary care provider (PCP)), competing comorbidities which may require more immediate attention, as well as social determinants of health which impede stability including unemployment, unstable housing, lack of transportation, incarceration, and ongoing substance use. Providers are often unwilling to prescribe HCV treatment to even former PWID because of concerns related to ongoing substance use, low adherence, and the potential risk of reinfection [ ]. Some studies have suggested that physicians have suboptimal knowledge regarding HCV and its treatment which can impact screening and referral to care. Additional structural barriers include insufficient locations where HCV testing can be performed and where treatment can be delivered [ , ]. There is also a dearth of providers who feel competent to treat HCV, as well as inadequate numbers of patient navigators dedicated to linking HCV-infected PWID and others into care and treatment. Finally, HCV care has historically been delivered in specialist settings which means it is segregated from other services that PWID utilize including primary care, opiate substitution therapy, and HIV care ( Fig. 6.2 ).
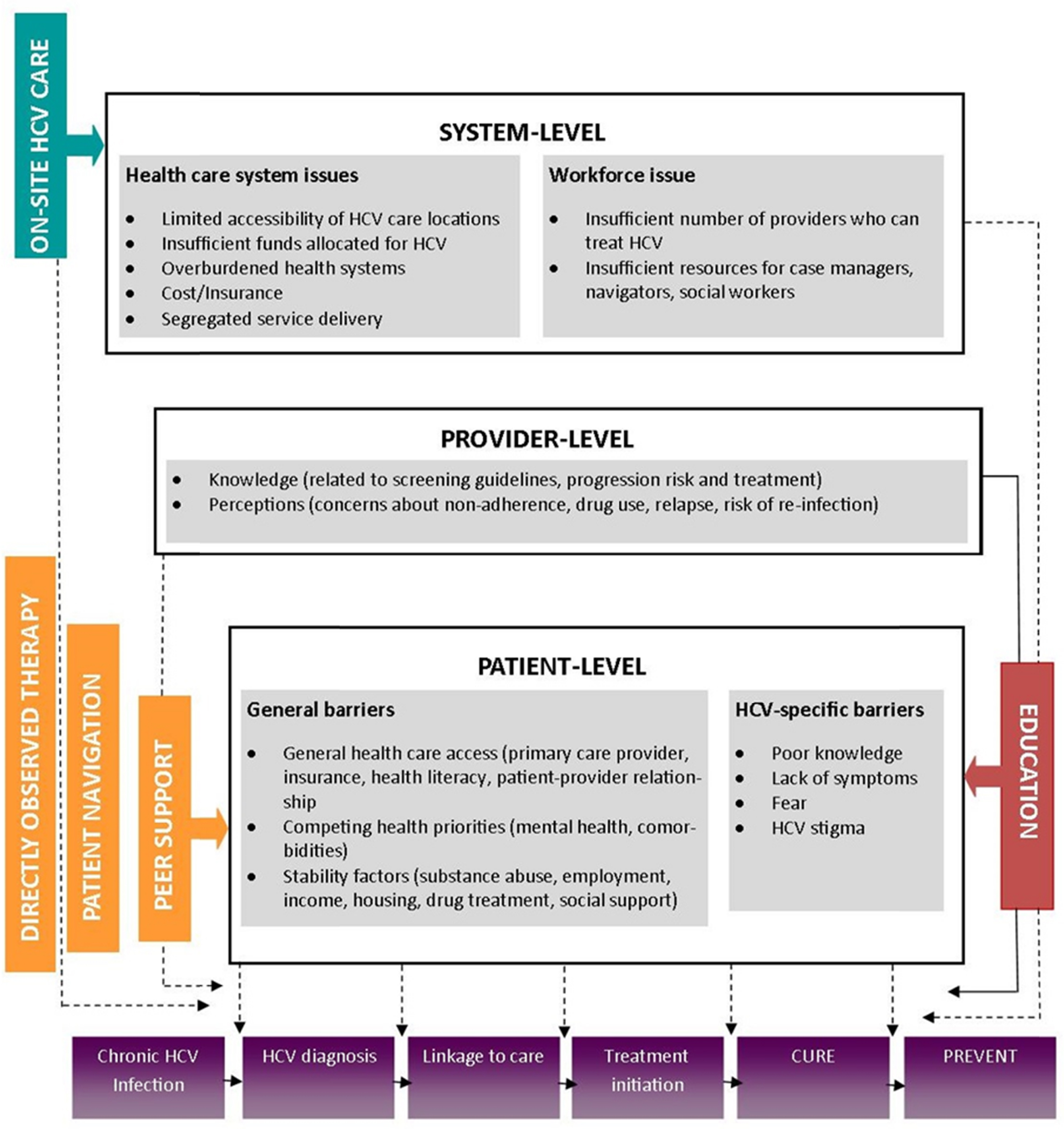
Cascade of care and Co-located models of care
The “cascade of care” model, also known as “care continuum,” is frequently used when the course of a medical condition has distinct, measurable endpoints, such as diagnosis, linkage to care, and treatment [ ]. Critical stages of the cascade of care for HCV may involve (1) screening for initial HCV infection, (2) HCV RNA confirmatory testing, (3) staging the degree of liver fibrosis, (4) engagement in care and HCV treatment, (5) and finally achieving a SVR (i.e., undetectable viral load 12 weeks after treatment completion) [ ]. This model provides a framework for clinicians, public health officials, and stakeholders to monitor a given disease at population level, and identify gaps in the delivery of care [ ].
Despite life-saving HCV medications, the HCV cascade of care remains dismal for PWID in this country. Lack of testing services, especially at alcohol and other drug centers, lack of linkage services, an inadequate number of treatment providers, and expensive HCV medications with restrictive state insurance policies have all led to limited access to HCV care for PWID, negatively impacting the care continuum [ ].
Screening
The first step toward improving HCV treatment outcomes among PWID is widespread screening. To date, screening strategies have focused on either risk-factor screening and/or age-based screening of the baby boomer population (those born between 1945 and 1965). In March 2020, the United States Preventative Services Task Force updated its recommendation, which now specifies that HCV screening is recommended for all adults (ages 18–79). This recommendation is based in part on the evolving bimodal age distribution of HCV infection, including both baby boomers and younger PWID, who have transitioned from oral prescription pain killers to injection of opioids. The AASLD/IDSA HCV Guidance Panel and CDC have also endorsed one-time HCV screening for all adults in the United States [ ]. Increasing incident HCV infections have paralleled the current opioid epidemic, yet many PWID continue to go unscreened. Consequently, updated guidelines are a welcome addition in order to increase screening rates.
Multiple innovative programs and models have demonstrated that co-located models of screening and care for HCV are feasible at community health centers as well as alcohol and drug treatment programs. In a community-based testing program in San Diego, HIV testing counselors were cross-trained on HCV testing and counseling, and between April 2013 and September 2013 conducted 453 rapid tests at three clinic sites and six residential alcohol and drug treatment programs. Overall, 94 (21%) of tested patients were HCV antibody positive, 67% fell within the birth cohort of 1945–1965, and 70% of patients were linked to care [ ].
HCV linkage to care
Though screening must improve, linkage to HCV care for those PWID already diagnosed and aware of their infection remains a primary barrier to receiving life-saving treatment. The CHAMPS study investigated novel interventions (peer navigators and cash incentives), as compared with usual care, to increase the rate of HCV treatment initiation and cure in a population of persons with HIV/HCV coinfection who use drugs [ ]. Overall, 110 of 144 (76%) of participants initiated treatment with ledipasvir/sofosbuvir. The initiation rate was higher in persons randomized to peers (83%) or cash incentives (76%) compared with usual care (67%)—however, these differences did not reach statistical significance ( P = 0.11). Among the participants who initiated treatment, SVR rates were high, achieved by 91% of participants. Importantly, PWIDs who attended their first linkage to care appointment without rescheduling were more likely to initiate treatment and obtain cure. Those who were unemployed were more likely to benefit from peers. These data stress the importance of expedited linkage to care for PWID and interventions that are tailored to each individual’s needs. Furthermore, active alcohol consumption and drug use did not lower treatment initiation rates, and SVR remained high.
For PWID outside of the healthcare system, linkage to care has been even more difficult [ ]. Though the data are sparse, linking PWID from needle and syringe programs and from correctional settings could be beneficial and should be actively implemented and studied.
Co-located care
Co-located care models in which HCV care is provided in the same settings as substance use treatment programs may be one of the most effective approaches to deliver HCV care among PWID. Co-locating HCV treatment in other settings where PWID are already accessing services, such as primary care clinics, syringe service programs, or jails and prisons, has also been shown to be effective. Components of care in these clinical settings are varied but may include (1) HCV treatment to prevent transmission (i.e., treatment as prevention), (2) provision of MOUD to reduce risk of HCV reinfection or infection of other infectious diseases, (3) access to HIV preexposure prophylaxis (PrEP), (4) behavioral services to address psychiatric symptoms, and (5) harm reduction services (i.e., syringe service programs and naloxone).
Co-located HCV care in opioid treatment programs (OTPs)
OTPs may be an ideal setting to treat PWID with chronic HCV given that OTPs are staffed by a multidisciplinary team able to provide medical, psychiatric, and addiction treatment, adherence support, peer support, group education and support, and potentially directly observed HCV therapy (DOT).
Many real-world cohort studies have shown high SVR rates, similar to registration trials, among those treated at OTPs in the DAA era [ ]. Many of these studies also took advantage of daily methadone distribution to offer DOT for HCV medication. The potential significance of such co-located care models is extensive given that nationwide, over 375,000 patients receive methadone or buprenorphine from approximately 1500 OTPs and conservative estimates suggest that over 60% of PWID in OTPs are infected with HCV. Understanding how best to incorporate HCV models of care within OTPs is important.
The PREVAIL study was a randomized controlled trial (RCT) conducted within three OTPs in Bronx, New York, to determine whether DOT and group treatment (GT) were more effective than self-administered individual treatment (SIT) in promoting adherence and achieving SVR among PWID receiving OAT [ ]. Among 150 PWID, 65% of participants had a positive illicit urine drug screen 6 months prior to HCV treatment. Overall SVR was high at 94%; 98% in the DOT group, 94% in the GT group, and 90% in the SIT group ( P = .152), no difference between study arms. Overall daily adherence was 78% and was greater among participants randomly assigned to DOT (86%) than those assigned to SIT (75%; P = .001). This study demonstrates that PWID receiving onsite HCV treatment at an OTP can achieve high SVR rates, despite ongoing drug use. Though it did not impact SVR, adherence was higher in the DOT group, indicating that leveraging the OTP infrastructure to offer DOT may be beneficial for those most at risk of nonadherence. These data support the widespread application of co-location of HCV treatment within substance use disorder treatment centers.
Co-located HCV treatment in primary care
Co-locating HCV treatment in primary care clinics is another model that can aid in expanding HCV treatment to PWID, circumventing the dearth of specialty care for many locations throughout the United States. Even in the arduous era of interferon, the ECHO study (RCT comparing SVR rates of participants treated via specialty care vs. primary care in rural clinics and prisons) proved that primary care physicians could successfully treat patients at comparable rates as specialists after telemedicine training [ ]. Nationally, there are over 1200 federally qualified health centers serving over 20 million marginalized patients, many with HCV prevalence rates higher than the national average [ ]. Though DAAs are highly efficacious, we will need to decentralize HCV treatment in order to increase access to care to these life-saving medications for the three to four million persons living with HCV in the United States.
The ASCEND trial evaluated the efficacy of HCV treatment managed by three provider types—nurse practitioners (NPs), primary care providers (PCPs), and specialists—set within a real-world, urban population. Patients with chronic HCV infection were assigned to receive treatment with ledipasvir and sofosbuvir from an NP (n = 150; 25%), a PCP (n = 160; 27%), or a specialist (n = 290; 48%). Overall, patients predominantly were male (69%) and black (96%); 40% had a fibrosis score 3/4, 25% had HIV coinfection, and 15% had active drug use.
There were no differences in SVR rates among the three provider types: NPs 89%; PCPs 87%; and specialists 84%. Importantly, adherence to appointments was higher among patients treated by NP and PCPs as compared with those treated by specialists, indicating that this relationship may improve engagement while on HCV treatment or after-treatment care for those that may need it most, such as PWID.
In a cohort study conducted at a primary care clinic in the Bronx, New York, SVR rates were found to be high for HCV-infected patients (n = 89) initiating DAA treatment [ ]. SVR rates were 95% (n = 41/43) for those who did not use drugs and 96% (n = 44/46) for patients actively using drugs and/or receiving OAT ( P = .95). These data support both treatment of PWID and using primary care clinics as important settings in which to engage patients who otherwise may not reach specialty care.
With the goal of HCV elimination, the United States will need to engage a larger pool of those living with HCV by task-shifting to nonspecialists, particularly for hard-to-treat populations such as PWID. Data support the reversal of state polices that prohibit nonspecialists from delivering HCV care to their patients, and treating HCV in these community-based centers will be key to the rapid scale-up of treatment and cure.
Similar models of collocated care may also be effective in increasing HCV treatment uptake in needle and syringe programs and correctional facilities, including jails and prisons, but more data are needed to support the implementation of best practices.
HCV treatment adherence
For decades, HCV treatment has been neglected for PWID due to provider’s misconceptions regarding medication adherence. Of note, recent studies have demonstrated that PWID can achieve optimal adherence rates. In the SIMPLIFY study, a multicenter study involving active PWID, the average daily adherence to therapy was 94%, and 94% of PWID achieved HCV cure. In the PREVAIL study, the HCV daily adherence rate among PWID receiving OAT was lower (74%), yet SVR rates remained high (94%). Furthermore, a recent meta-analysis found that adherence rates were similar between PWID and non-PWID in the era of DAAs (adherence defined as >90%). There were no differences in SVR rates between those defined as adherent and those that were not. This may indicate that, in the era of DAAs, a 90% adherence threshold is too high to predict failure. From these studies, it is evident that most PWID can achieve high rates of DAA adherence, and that nonperfect adherence can still lead to high SVR rates. Overall, the decision to exclude PWID from HCV treatment should be avoided even if patients have poor adherence during HCV treatment, and emphasis on treatment completion should be encouraged.
HCV reinfection
HCV antibody does not provide immunity from reinfection. HCV reinfection is defined as a positive HCV RNA test after a documented SVR at least 12 weeks after the completion of HCV treatment [ ], and overall estimates indicate that reinfection rates are low. A recently published study using data from the British Columbia Hepatitis Testers Cohort [ ], which includes all individuals tested for HCV or HIV at the British Columbia Center for Disease Control Public Health Lab from 1990 to 2010, is one of the largest HCV cohorts reporting reinfection to date. Among 2225 individuals who achieved SVR following HCV treatment, the overall reinfection rate was (0.48/100 PY) suggesting low rates of HCV reinfection. Higher HCV reinfection rates were reported among PWID (1.14/100 PY) and persons with HIV infection (2.56/100 PY), yet reinfection rates still remained relatively low.
Now, there are increasing data available on reinfection rates for PWID in the era of DAAs, and reinfection rates continue to be low [ ]. For example, the reinfection rate found in the SIMPLIFY study conducted among active PWID with recent IDU was found to be 2.6/100 PY [ ]. In the PREVAIL study conducted among PWID on MOUD, the incidence of reinfection reported was even lower (1.22/100 PY) [ ], which mirrors findings from earlier studies indicating that receiving MOUD reduces HCV reinfection risk among PWID [ ]. Importantly, all reinfections in this study were among participants reporting ongoing IDU, thus emphasizing the need for “wrap around” harm reduction services in conjunction with HCV treatment programs in PWID.
In a meta-analysis of 35 studies which included interferon-based regimens as well as DAAs, the overall rate of HCV reinfection was 6.2/100 person-years (95% CI 4.3–9.0) among people recently injecting drugs, 5.9/100 person-years (95% CI 4.1–8.5) among people with any recent drug use (injecting or non-injecting), and lowest, 3.8/100 person-years (95% CI 2.5–5.8) among those receiving MOUD [ ]. There were no significant differences in reinfection rates between people treated with interferon-based versus DAA therapy, and overall reinfection was reported to be relatively low.
Given the increasing evidence that reinfection rates remain low for PWID post SVR in the era of DAAs, concern for reinfection should not be used as a reason to withhold therapy from people who use drugs. Instead, target interventions to lower reinfection, such as MOUD and Syringe Services Programs (SSPs), should be offered in conjunction with HCV treatment; as well as regular posttreatment HCV assessment for early detection of reinfection and nonstigmatized HCV retreatment. If we are to approach HCV elimination, we must proactively treat the population most at risk for transmitting the virus.
The Hepatitis C Real Options (HERO) study is an ongoing, randomized study aimed at investigating the effectiveness of different models of curative HCV treatment among PWID. The HERO study is the largest prospective study to date (754 PWID) and will have follow-up over a 3-year period to determine reinfection [ ]. The HERO study has the potential to help identify specific risk factors for reinfection among active PWID which can be used for harm reduction efforts.
Treatment as prevention
Treatment as prevention is proven strategy to reduce transmission in HIV-infected persons [ ] and is an emerging concept in HCV. Mathematical models suggest that HCV could be significantly decreased to near elimination levels of <5% in communities with HCV prevalence <50% by providing DAA therapy among populations with the highest transmission risks, namely PWID and men who have sex with men [ ]. An example of treatment as prevention is the TraP HepC project in Iceland, which was initiated in 2016. All HCV RNA positive persons were offered DAA therapy, and among the 722 persons who initiated treatment SVR was achieved in 89%. Early reports indicate that universal access has now led to a marked reduction of HCV prevalence among PWIDs and the incarcerated in Iceland [ ]. A similar pattern of reduced HCV infection was also found to be true in a population of HIV/HCV coinfected MSM in the Netherlands. Unrestricted DAA availability after 2016 was followed by a 51% decrease in acute HCV infections among HIV-positive MSM, while other infections related to sexual activity increased during the same time period [ ]. Proactive treatment of populations most at risk of transmission, such as PWID, will be needed to reach HCV elimination targets.
Another innovative way to reduce the risk of HCV transmission as well as reduce deaths from opioid use involves HCV/HIV testing in combination with overdose prevention and response training at outreach facilities, and in a study done in Bronx, New York, there was high participant acceptability of such an intervention [ ]. SSPs and MOUD programs are extremely valuable tools in preventing HCV transmission in PWID. In a Cochrane review of such programs, the combination of SSPs and MOUD was associated with the greatest reduction in HCV transmission [ ].
Policy issues and restrictions
The implementation of new and innovative drug policies in the United States could facilitate a reduction of HCV among PWID in the United States. A clear example is Portugal where decriminalization of the purchase, possession, and consumption of drugs in 2001 coincided with a significant decrease in the number of people infected with HCV, as well as other infectious diseases related to drug use (i.e., HIV) [ ].
Overall, a wide adoption of evidence-based strategies could help mitigate HCV risk and improve treatment among PWID, yet their implementation in the United States is scarce and partially obstructed by the current policies. There remain restrictions by payers on who can be treated; and restrictive criteria in Medicaid programs currently exclude persons with lower stages of liver fibrosis, persons actively using drugs, and restrictive prescriber eligibility, and these policies were largely driven by the profoundly expensive HCV medications [ ]. The National Viral Hepatitis Roundtable and the Center for Health Law and Policy Innovation of Harvard Law School launched in October 2019 an update to “Hepatitis C: Sate of Medicaid Access,” an interactive project grading all 50 state Medicaid programs, as well as the District of Columbia and Puerto Rico, according to access to curative HCV treatments for HCV, the nation’s deadliest infectious disease. Although Medicaid access to HCV antiviral medications has improved over the last few years, more than half of all state Medicaid programs still impose some restrictions on provider type, fibrosis level, and illicit drug use.
Another example of policy that supports treatment scale-up to achieve elimination is Australia’s National Drug Strategy. In 2016, the Australian Government offered unrestricted access to DAA therapy with no restriction based on liver disease stage, drug and alcohol use, and incarcerated status. Additionally, any medical practitioner regardless of specialty could prescribe DAAs. This treatment-for-all, in combination with harm reduction strategies including government-funded syringe service programs and MOUD, could result in HCV elimination in Australia. As a consequence, recent mathematical modeling studies have proposed that Australia will meet the WHO targets of HCV elimination by 2028 [ ].There is evidence that scaling-up HCV treatment to 80% of the PWID infected could reduce prevalence HCV among PWID by at least 45% [ ]. Unfortunately, neither HCV testing nor care are routine practices implemented in North America [ ].
Cost-effectiveness studies
Treating HCV among PWID is highly cost-effective independent of the type of regimen provided. For example, studies conducted with older generation therapies (e.g., pegylated-interferon and ribavirin) were shown to be cost-effective among PWID [ ], despite the considerable side effects and low cure rates of interferon-based regimes. New DAA-containing regimens are cost-effective in various adult populations [ ]. A recent meta-analysis reviewing 92 results from 10 studies evidenced that DAA treatment is cost-effective at a $100 000/QALY threshold, independent of the presence of cirrhosis and prior treatment history [ ]. In the PREVAIL study, investigators analyzed the cost-effectiveness of SIT, GT, and DOT for HCV in OAT programs using trial results. GT and DOT models were found to be cost-effective alternatives to SIT, and these interventions could be adopted widely at OAT programs nationwide.
Conclusions
PWID have a high prevalence of chronic HCV and are a major source of new infections within the community. Highly effective and well tolerated, interferon free, all oral and pan-genotypic regimens have revolutionized the HCV treatment paradigm. These regimens are equally effective in PWID as they are in the general population. However, the majority of PWID in the United States have not initiated treatment and therefore have not achieved SVR. SVR is tantamount to HCV cure and leads to a significant improvement in hepatic and all-cause morbidity and mortality. HCV reinfection after SVR in PWID is low, and can be decreased even further with “wrap around” services including SSPs, MOUD, and co-located models of care. If DAA scale-up in PWID is supported by policy makers and payers, HCV eradication may become a reality.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree