Introduction
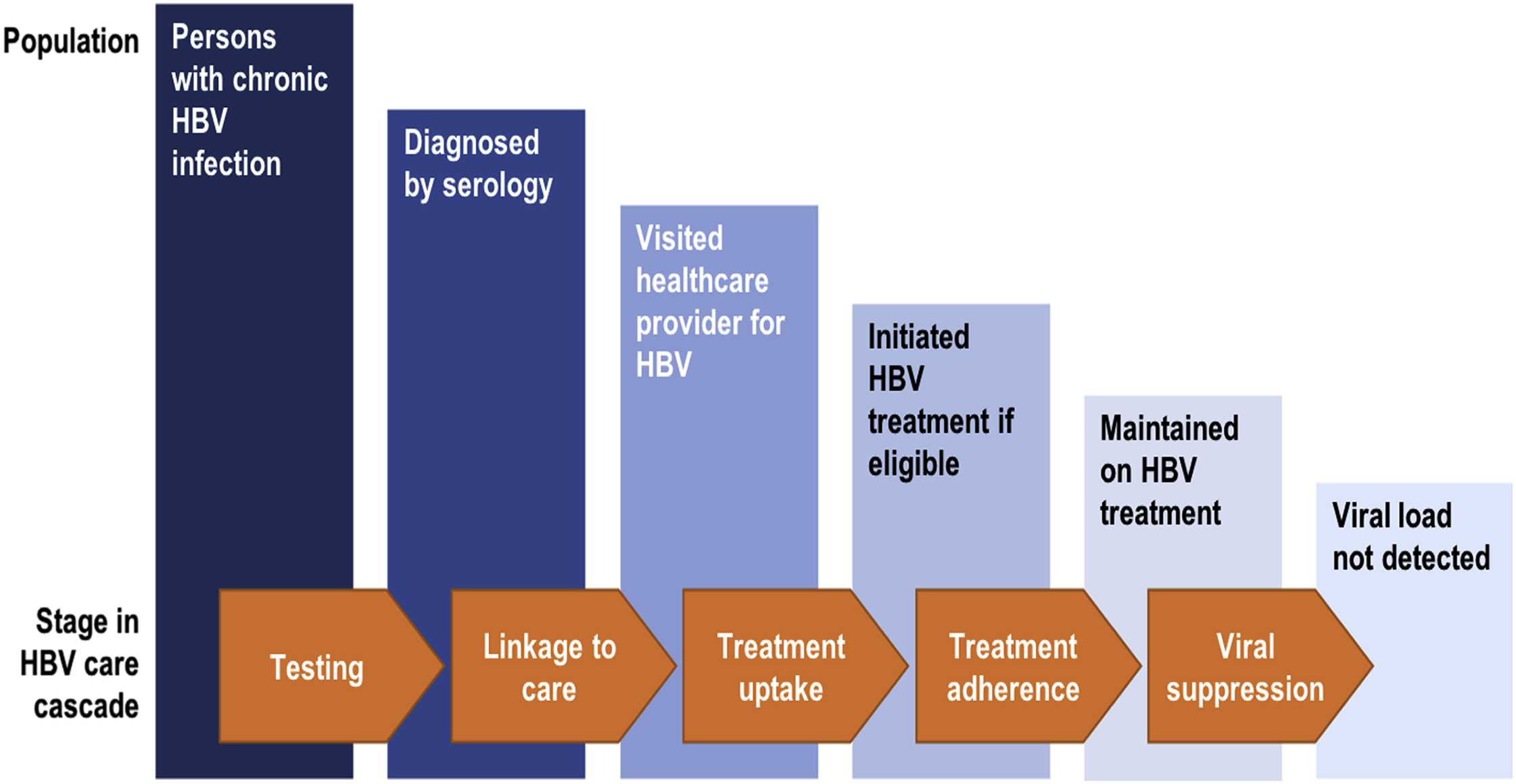
Chronic hepatitis B virus (HBV) infection affects over 240 million individuals worldwide [ ]. Detection of the disease is dependent on active surveillance and testing by healthcare providers. Due to the high risk of transmission, the Center for Disease Control (CDC) recommends HBV screening in all individuals with opioid use disorders [ ]. Prompt identification of chronic infection and vaccination of susceptible persons at initial point of contact with the healthcare system is a priority. HBV diagnosis and prevention strategies should be coupled with effective harm reduction programs [ ]. Safe and well-tolerated oral antiviral therapy is available and is highly efficacious at suppressing viral replication and ameliorating long-term complications; however, there is at present no cure [ ]. Successful navigation of chronically infected individuals through the HBV care cascade ( Fig. 7.1 ) is critical to reduce HBV-related morbidity and mortality.
Natural history of hepatitis B virus infection
A heterogeneous disease, chronic HBV infection can manifest in many ways, from long-term inactive disease with little impact on life expectancy to severe acute hepatitis that leads to liver failure and death in the absence of liver transplantation. Risk of chronic HBV infection following exposure differs by age. Spontaneous resolution of hepatitis B after exposure is high among healthy adults but lower in adults who are immunocompromised.
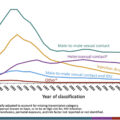
The course of chronic hepatitis B is characterized by variable periods of high viral replication and active hepatitis (liver inflammation and injury) and more quiescent periods with low viral replication and inactive liver disease ( Fig. 7.2 ). The initial immune-tolerant phase (high viral replication, low activity) is typically absent or very short when infected in adulthood versus lasting decades in those infected as neonates or youngsters. Progression through the different phases of infection is not linear and all adults do not experience all phases during their lifetime. Roughly 30% of individuals with chronic HBV infection have an active disease phenotype marked by fluctuating periods of viral activity and subsequent hepatic inflammation (elevated alanine aminotransferase [ALT]) and intervening periods of viral quiescence [ ]. Over time, usually on the order of decades, the repetitive injury to the liver leads to the formation of liver fibrosis and ultimately cirrhosis in about 15%–25%. On the other hand, about 20%–30% of chronically infected individuals are inactive carriers, with low HBV DNA and normal hepatic enzyme levels, and possess a much more benign course of disease with minimal risk of cirrhosis over their lifetime unless coexisting liver diseases are present. Risk of HBV-related complications of cirrhosis and hepatocellular carcinoma (HCC) positively correlates with the level of HBV viremia.
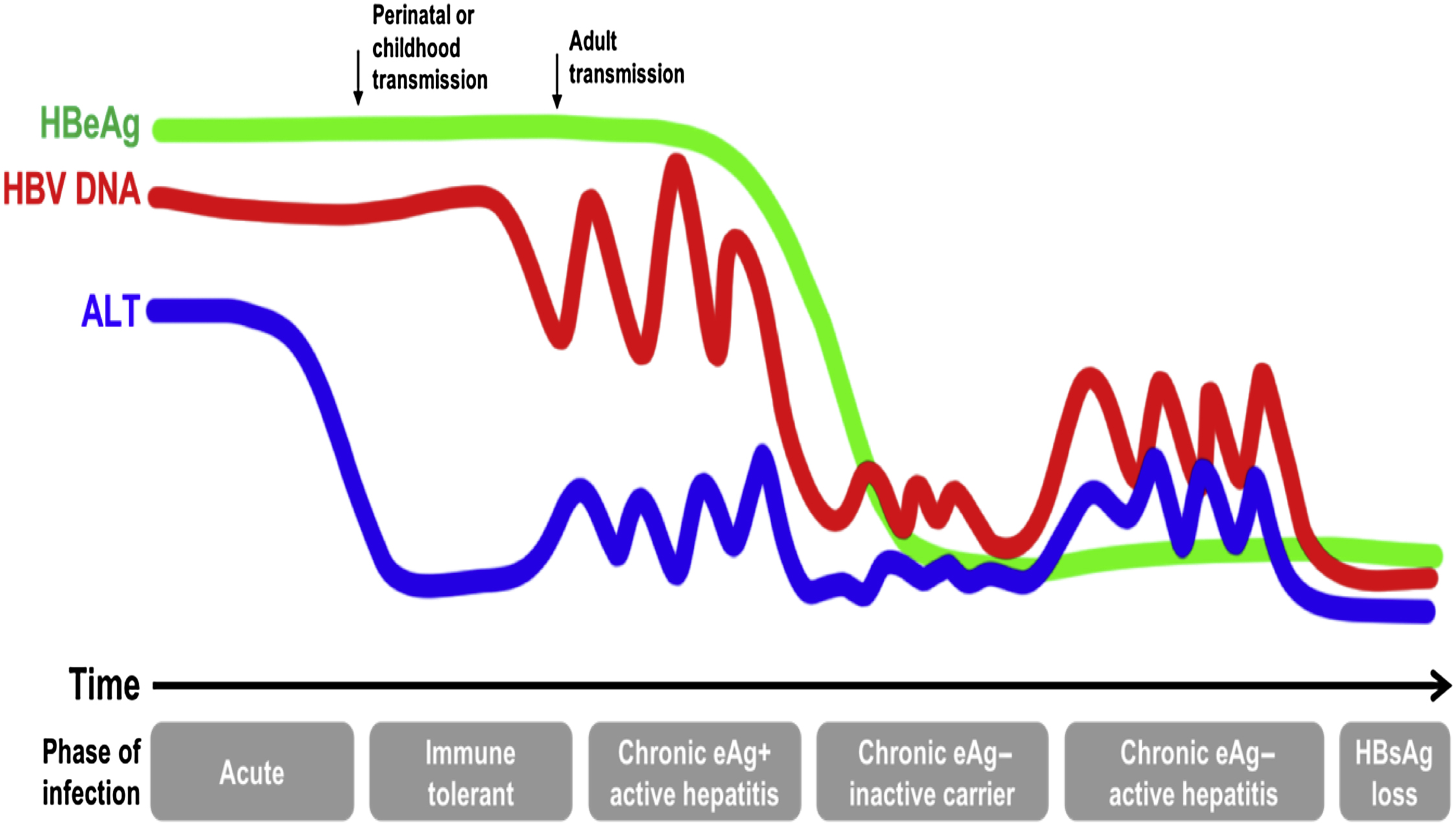
Once chronic infection is established, spontaneous resolution is highly infrequent at all ages, with hepatitis B surface antigen (HBsAg) loss occurring at a rate of ∼1% per year [ ]. Furthermore, unlike other hepatotropic viruses, HBV integrates its DNA into hepatocytes and can be directly carcinogenic, leading to the development of liver cancer in the absence of fibrosis. Screening for liver cancer, therefore, is a critical aspect of clinical management in specific subpopulations of chronically infected individuals. Thus the vast majority of persons with chronic hepatitis B will require management of their infection over their lifetime, with the need for regular monitoring to determine indications for and duration of therapy. These considerations present unique challenges in the care of HBV-infected individuals with opioid use disorders.
Hepatitis B virus infection epidemiology, with focus on persons who inject drugs
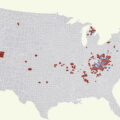
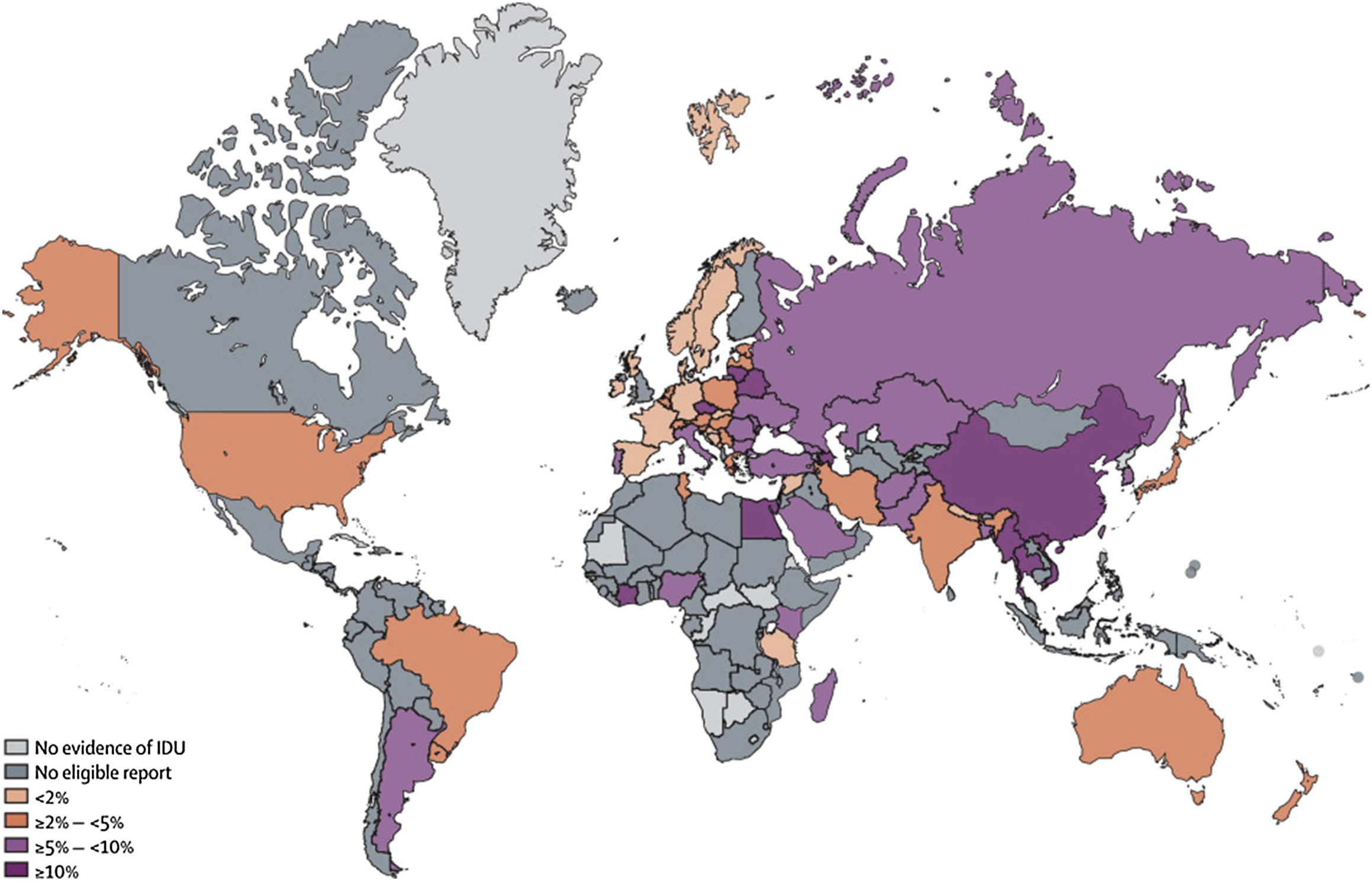
Globally there are an estimated 15.6 million persons who inject drugs (PWID) in the adult population, [ ]. Substantial regional variation exists with respect to global HBV prevalence among PWID, reflecting the HBV endemicity of the region ( Fig. 7.3 ). While overall approximately 9.1% of PWID are HBsAg positive, the proportion is highest (20%) in East and Southeast Asia and lowest in Latin America and Western Europe (2.8% and 3.2%, respectively). The estimated HBV prevalence among PWID in North America is intermediate at about 5% [ ].
Injection drug use (IDU) accounts for the majority of HBV infections among those born in the United States. Unlike hepatitis C virus (HCV), prevention of HBV infection is achievable through widespread availability of a highly effective HBV vaccine. However, those born or infected before the introduction of universal birth-dose vaccination in the United States in 1991 remain at risk for acquisition of HBV and subsequent complications [ ]. In fact, the rise of opioid use disorders has been an important vector for the ongoing spread of HBV. In the past decade, acute HBV outbreaks among the IDU population have been reported in the Appalachian states and have signified a shift in the incident disease burden from urban to nonurban communities [ ]. Among those with opioid use disorders, transmission most commonly occurs through sharing needles, with sexual transmission being a secondary risk factor, which can be related to unsafe behaviors such as the exchange of sex for drugs [ , ]. In addition, incarceration occurs at higher rates among opioid users and is an established risk factor for HBV acquisition [ ]. Coinfection with human immunodeficiency virus (HIV), HCV, and hepatitis delta virus (HDV) is also more frequent among opioid users because of the shared routes of transmission, and these coinfections increase the risk of progression to end-stage liver disease [ , ].
Acute hepatitis B
Acute HBV infection may be accompanied by nonspecific symptoms such as fatigue, myalgias, nausea, and uncommonly jaundice, while symptoms are typically absent in those with chronic infection. Although usually self-limited, acute HBV infection can have severe consequences. Acute or fulminant liver failure due to HBV, defined as coagulopathy and encephalopathy within 8 weeks of symptom onset, occurs in 0.1%–0.5% of acute cases and spontaneous recovery without liver transplantation is infrequent even with prompt administration of antiviral therapy [ ]. Factors associated with a higher likelihood of acute liver failure among acutely infected PWID include concomitant alcohol use, methamphetamine use, HBV genotype D, and certain HBV mutations [ ].
Epidemiologic data on acute HBV incidence in opioid use disorders are sparse and lacks disaggregation by drug type. Outbreaks are commonly related to sharing of injection equipment including filters and water used to prepare/rinse syringes [ ]. An enhanced population-based surveillance strategy employed by six states (Colorado, Connecticut, Minnesota, Oregon, Tennessee, and New York) identified 2220 acute HBV cases between 2006 and 2011 with an average annual incidence rate of 0.86 per 100,000 persons [ ]. There was a decline in cases over time in all sites except Tennessee where the incidence peaked at 2.26 per 100,000 persons in 2011. The majority of cases were US-born, white, male, and aged 30–49 years (mean, 44 years). IDU, particularly of heroin, is the main driver for acute HBV infection in US regions with increasing incidence [ ]. Between 2006 and 2013, over 3000 cases of acute HBV infection were reported to CDC from the Appalachian states of Kentucky, Tennessee, and West Virginia alone, representing an increase of 113% in these three states. Over this period, the proportion of cases increased among those of white race, aged 30–39 years, and in nonurban counties (defined as population under 50,000). Report of IDU as a risk factor for HBV transmission increased as well (53% between 2006 and 2009 vs. 75% between 2010 and 2013), mirroring a rise in admissions for prescription opioid and heroin use disorders among young adults in these three states [ ]. The escalating incidence of acute HBV in the 30- to 49-year age group may reflect susceptibility due to a lack of inclusion in routine or catch-up HBV vaccination and a growing engagement in risky behaviors. Outbreaks are reported in other states, with Massachusetts reporting a 78% increase in acute HBV cases in 2017 and North Carolina seeing a 56% increase between 2014 and 2016 [ ], highlighting the need for broader vaccination efforts.
Natural history studies reveal that ∼90% of adults with acute HBV infection will clear HBsAg within 3–6 months, but delayed clearance of up to 2 years has also been described [ ]. In a prospective study of acute hepatitis from Europe (most reported sex as a risk factor), the median time to HBsAg clearance was 67 days (interquartile range [IQR], 32–132 days) and median time to hepatitis B surface antibody (anti-HBs) >10 IU/L was 109 days (IQR, 49–169.5 days) [ ]. In another study from Japan, HBV genotype A was found to be associated with delayed clearance of HBsAg (mean, 6.7 vs. 3.4 months), with 23% clearing HBsAg 6 months after the onset of acute hepatitis [ ]. By definition, chronic HBV infection means the presence of HBsAg for 6 months or more, but in the case of documented acute HBV infection, a longer period of follow-up may be needed to establish chronicity with certainty.
Resolved and occult hepatitis B
In the United States, approximately 3.9% of the population, an estimated 10.8 million individuals, has evidence of resolved HBV infection [ ]. Resolved HBV infection is very common in PWID exposed in adulthood. This reflects the high rate (>95%) of spontaneous clearance of HBV infection after acute infection among healthy adults. Risk of HBV exposure increases with the length of IDU. Therefore resolved HBV infection can be found in as high as 95% of those with greater than 30 years of use [ ]. Although resolved infection is generally regarded as benign, the presence of positive hepatitis B core antibody (anti-HBc) has been associated with 1.3-fold increased all-cause mortality in the United States, which may be attributable to coexisting high-risk behaviors rather than liver-related complications [ ]. However, reactivation of HBV infection (reversion to HBsAg positivity or reappearance of HBV DNA) in the setting of immunosuppression is an important risk in those with resolved hepatitis B. The risk not only is highest (>10%) with the use of anti-CD20 therapy (i.e., rituximab, ocrelizumab) and hematopoietic stem cell transplantation but also exists with exposure to cytotoxic chemotherapy, biologics, and high-dose corticosteroid therapy (1%–10%) [ ].
Occult HBV infection is defined as absent serum HBsAg, but presence of serum anti-HBc and detectable HBV DNA, albeit in very low concentrations. Theories for lack of circulating HBsAg include gene mutations that lead to HBsAg nondetectability and, more likely, robust immune control of virus leading to low HBV replication. High prevalence of this form of HBV infection has been reported among PWID, although prevalence measures are dependent on the sensitivity (lower limit of detection [LLD]) of the assay used. Among 188 HCV-positive PWID in Baltimore, Maryland, HBV DNA was detected in 45% using an enhanced PCR assay (LLD 15 copies/mL) compared with 0% when using an older COBAS PCR assay (LLD 200 copies/mL) [ ]. In another study from Taiwan, 41% of 301 HBsAg-negative PWID had positive results for serum HBV DNA (mean, 4.0 log copies/mL) [ ]. No mutations were found in 20 randomly selected samples. In both studies, occult infection was associated with older age, likely a reflection of duration of IDU and cumulative HBV exposure. While treatment is not indicated, occult infection has been linked to infrequent events of HBV transmission, fibrosis progression (particularly with HCV coinfection), HCC, and reactivation [ ].
Chronic hepatitis B
Current US estimates of HBV-infected individuals range from 0.8 to 2.2 million, many of whom are immigrants from endemic regions of Asia and sub-Saharan Africa [ ]. The seroprevalence of chronic HBV infection (positive HBsAg) has been relatively stable over the past two decades among US adults at 0.3%, based on the National Health and Nutrition Examination Survey (NHANES), although likely an underestimate because of the exclusion of military, incarcerated, and homeless population from sampling [ ]. Population-based estimates of HBV prevalence among self-reported PWID in NHANES are not available and other estimates of HBsAg seroprevalence among PWID have ranged from 3.5% to 20%, attributable to the study design (i.e., convenience sampling, recruitment site) and cohort differences [ ]. The higher prevalence of resolved versus chronic infection reflects the considerable burden of HBV exposure coupled with a high likelihood of clearance.
Data suggest that the trend in HBV exposure over time among PWID has largely declined. The proportion of anti-HBc-positive cases in cross-sectional sampling of IDU individuals in the Urban Health Study in San Francisco decreased from 68% in 1987 to 45% between 1998 and 2000 [ ]. Increased uptake of HBV vaccination is one explanation, as only 1% had serologic evidence of vaccination in the early period compared with 12% in the later period. Similarly, in a cross-sectional sampling of PWID residing in Seattle, anti-HBc prevalence declined from 43% to 15% between 1994 and 2004, with coexisting increases seen in self-reported needle-exchange use, condom use, and vaccination uptake [ ].
HBV-related mortality
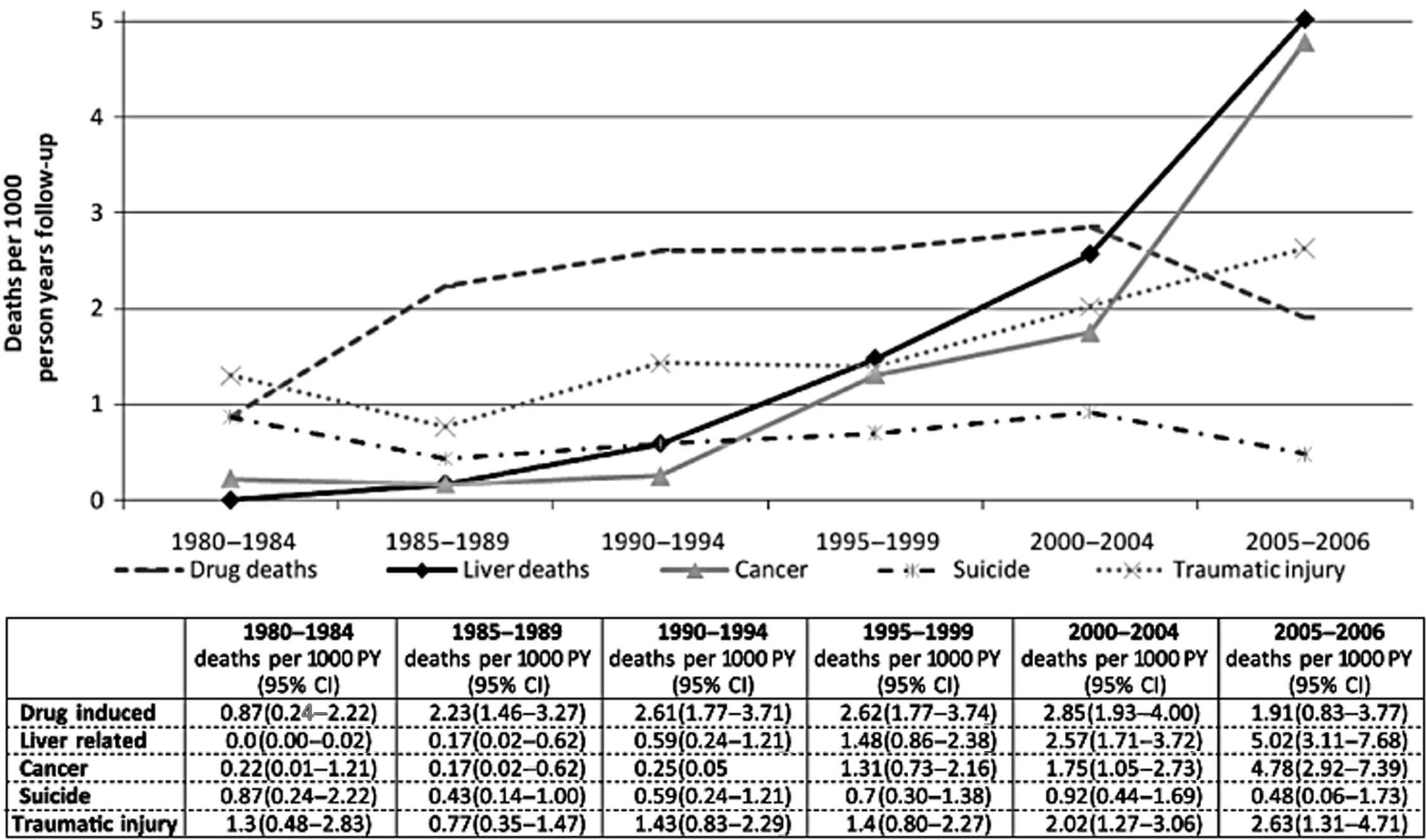
About 1800 individuals die from the sequelae of chronic HBV infection yearly in the United States, including HCC and complications of cirrhosis [ ]. Mortality rates are much higher among those with opioid use disorders and IDU than the general population; most commonly early deaths are from drug overdose, suicide, traumatic injuries, and complications of HIV [ ]. The risk of liver-related mortality is estimated to be 17-fold higher among PWID as compared with the general population. Viral hepatitis accounts for three-quarters of those who die from liver disease, with chronic alcohol use contributing in up to 40% [ ]. Historically, the impact of HBV infection on life expectancy among IDU individuals was much less than that of chronic HCV infection [ ]. But the long latency of viral hepatitis means liver disease has emerged as a major contributor to mortality only as opiate-dependent populations have aged, particularly as life expectancy with HIV infection has lengthened with improved therapies. In a large Australian cohort of PWID, liver disease was the underlying cause of or contributed to 17% of deaths over 30 years; there was a clear uptrend over time, with 0 deaths per 1000 person-years between 1980 and 1984 to 5 deaths per 1000 person-years between 2005 and 2006, overtaking death rates from overdose [ ] ( Fig. 7.4 ).
Hepatitis B and other coinfections in persons who inject drugs
Coinfection with HIV, HCV, and/or HDV is frequently encountered among those with opioid use disorders because of the shared means of transmission. Age at first injection, incarceration, men who have sex with men (MSM), and greater number of lifetime partners have all been identified as risk factors for coinfection [ , ]. The interplay of viral dynamics in those with coinfection can have significant bearing on clinical outcomes, most notably the risk of cirrhosis, HCC, and liver-related mortality.
Human immunodeficiency virus
HBV screening is recommended in all HIV-infected persons, and all PWID and MSM who are HBsAg-positive should be tested for HIV [ ]. Among HIV-infected cohorts, rates of coinfection with HBV can be as high as 20% in endemic regions compared with 5%–8% in nonendemic regions, related to the age of exposure and likelihood of chronicity [ , ]. In nonendemic regions, HBV infection may precede HIV infection because of the more efficient transmission of HBV. MSM are a much stronger determinant of HBV infection risk than IDU among HIV-infected adults in nonendemic regions [ ]. In comparison, IDU accounts for most cases of HCV/HIV coinfection. Concurrent HIV infection influences the natural history of HBV infection: those with lower CD4 counts are more likely to develop chronic infection after exposure, experience HBV reactivation, and develop cirrhosis and HCC [ ]. Conversely, HBV infection appears to have little impact on HIV natural history, with no differences seen in progression to AIDS and response to antiretroviral therapy between HIV monoinfected and HIV/HBV coinfected persons [ ]. Among HIV-infected PWID, HIV care models provide an ideal opportunity to integrate HBV testing, vaccination, and treatment.
Hepatitis C virus
Over 60% of HCV-infected adults in the United States have evidence of prior HBV infection while dual infection with chronic HBV is present in 6% [ ]. In one study performed in New York City, the strongest predictors of coinfection were Asian race, IDU, and the number of lifetime sexual partners. PWID were eight times more likely to have HBV/HCV coinfection than those who did not report IDU [ ] and simultaneous acute infection is more common [ ]. Dual infection with HBV and HCV substantially increases the rates of liver disease progression compared with monoinfection with either virus [ , ]. Coexisting HCV infection appears to induce suppression of HBV replication, but the specific mechanism underlying this phenomenon is still unknown. The complex interaction between the two viruses is evident in the context of HCV therapy where HBV reactivation has been reported during and after HCV treatment, leading to guideline recommendations to screen for HBsAg before the initiation of HCV direct-acting antivirals (DAAs) [ , ].
Hepatitis D virus
Unlike HIV and HCV, HDV is unique in that it requires HBV proteins for viral assembly and proliferation within a human host. HDV can be acquired either at the same time as HBV (acute coinfection) or in a host with established chronic HBV infection (superinfection). Acute superinfection carries an increased risk of acute liver failure and can be mistaken for an HBV flare. In about half of patients, HBV DNA levels are low when HDV RNA levels are high; thus the index of suspicion for HDV should be high when ALT levels are elevated despite a low level of HBV DNA [ ]. The estimate of HDV-infected persons in the United States ranges from 3% to 12%, with the accuracy of estimates limited by the relatively low uptake of testing in clinical settings [ , ]. However, much higher rates of HBV/HDV coinfection in the United States have been detected among PWID, ranging from 36% to 50% [ , ], which is comparable to rates in HDV-endemic regions such as Central Africa and Mongolia [ ]. The combination of HBV and HDV results in the most rapidly progressive form of hepatitis, with progression to cirrhosis within 2 years in 10%–15% of affected individuals and a threefold higher risk of HCC [ ]. The high prevalence of HDV among PWID and worse prognosis reinforce the need to screen for HDV infection among all HBsAg-positive persons.
Hepatitis B virus screening and diagnosis
Awareness and knowledge
Awareness of hepatitis B infection among PWID is glaringly low. Less than 40% of the adult US population are aware of their HBV infection and this percentage drops to 15% among PWID [ , ]. Concordance between self-reported and serologic evidence of HBV status, including immunization status, is very poor and self-report should not be relied on for estimates of disease burden or clinical decision-making. Between 46% and 95% of PWID without self-report of HBV infection had serologic evidence of exposure [ ]. Furthermore, knowledge regarding HBV transmission and risk factors is lacking. Among 493 inner-city PWID, only one-third were aware that there was no cure for HBV infection and 55% were aware that a vaccine was available [ ]. In comparison, over 85% knew there was no cure and no vaccine for HIV infection—a marker of the success of numerous HIV prevention programs that have been implemented in high-risk populations. In another survey of participants in a syringe exchange program, only 49% were aware HBV was transmitted through sharing needles despite 33% reporting testing positive for HBV [ ]. Increased knowledge on hepatitis was associated with known infection, substance abuse treatment, and less risky injection behaviors, suggesting good retention of knowledge after engagement with hepatitis-related healthcare systems and providers. With the rising burden of IDU in nonurban settings, a study found that PWID in urban (vs. nonurban) areas were not more knowledgeable despite increased access to harm reduction services [ ]. While most implementation studies in PWID have focused on the integration of HCV-specific patient education and testing into opiate substitution and drug treatment programs, these measures would likely produce similar success in boosting HBV awareness, testing, and vaccination (when appropriate) among PWID.
Screening
The initial test to screen for infection is a qualitative HBsAg test. In PWID, total anti-HBc and anti-HBs should also be tested at initial screening to determine past exposure and immune status, respectively. The gold standard test for HBsAg is a laboratory-based enzyme-linked immunoassay, which may not be ideal for those with opioid use disorders, who for many reasons may be unable or unwilling to follow-up on testing results.
Point-of-care (POC) tests are valuable alternatives in these populations, whereby results can be divulged at the time of testing and where convenient for the patient [ ]. For HBV in particular, POC testing for immunity is of equal significance to diagnosis, as subsequent delivery of vaccine has benefits. Currently, POC immunologic rapid diagnostic tests (high sensitivity and specificity in research studies) for HBV have yet to be approved by the US Food and Drug Administration (FDA) and are not clinically available. Capillary whole-blood dried blood spot (DBS) testing is an alternate test increasingly utilized in resource-limited settings because it allows for multiple tests to be performed on a single sample, enables easy collection (important for persons with difficult venous access) and transportation of samples, and has good test characteristics. The disadvantage of DBS testing is that it is not a POC test; that is, results are not immediate and a second consultation is still required for decision-making.
Diagnosis
Diagnosis is made on the basis of serologic markers of infection ( Table 7.1 ). If concern for acute infection exists (marked serum aminotransferase level elevation), the serologic test for diagnosis is a positive IgM-specific anti-HBc. Chronic infection is characterized by the persistence of HBsAg for at least 6 months. Those with a positive anti-HBc and anti-HBs with negative HBsAg have spontaneously cleared the infection and are immune. An isolated positive anti-HBc can represent occult infection (positive HBV DNA), resolved infection (either spontaneously or with treatment) with waned anti-HBs titers (negative HBV DNA), or a false-positive result, although the latter is very unlikely in PWID. Those with negative HBsAg and anti-HBc but positive anti-HBs are vaccinated, with anti-HBs titers ≥10 IU/L, signifying adequate immunity.
| HBsAg | Anti-HBc IgM | Anti-HBc total | Anti-HBs | Interpretation | Comment |
|---|---|---|---|---|---|
| − | − | − | − | Never infected | |
| − | − | − | + | Vaccinated | Immune if anti-HBs titers ≥10 IU/L |
| +/− | + | − | − | Acute infection | Can be anti-HBc positive only during window period |
| + | − | + | − | Chronic infection | HBsAg positive for at least 6 months |
| − | − | + | +/− | Resolved infection | Anti-HBs can be negative with waned titers Also seen with spontaneous or treatment-induced HBsAg loss in chronically infected people |
| − | − | + | − | Occult infection | Occult if low-level HBV DNA is detected May also be a false-positive result, particularly among individuals without risk factors Also seen with spontaneous or treatment-induced HBsAg loss without anti-HBs seroconversion |
Testing for hepatitis B e-antigen (HBeAg) and hepatitis B e-antibody (anti-HBe) helps determine the phase of infection and the level of HBV DNA at which treatment should be initiated (see the section Treatment of hepatitis B). Serial measurements of HBV DNA and ALT levels (performed typically every 3–6 months for the first year) are frequently necessary to establish the baseline phase of infection because of the fluctuating nature of HBV infection. Additional HBV studies such as HBV genotype and testing for the presence of HBV mutations are not recommended in routine clinical practice. Testing for coexisting HIV, HCV, and HDV infection should be performed. Although there is growing interest in the utility of quantitative HBsAg levels in classifying phase of infection, use of this serologic marker has not yet been standardized or incorporated into guidelines [ ].
Prevention: Hepatitis B virus vaccination
Safe and highly effective vaccines are available for HBV. The HBV vaccine, consisting of highly purified HBsAg protein, induces endogenous production of anti-HBs and stimulates memory T cells. There are three commercially available FDA-approved vaccines in the United States: Engerix-B (three doses; GlaxoSmithKline Biologicals), Recombivax HB (three doses; Merck & co), and Heplisav-B (two doses; Dynavax Technologies); the last vaccine was approved in 2017. Additionally, a three-dose combined hepatitis A virus/HBV vaccine, Twinrix (GlaxoSmithKline Biologicals), which includes the Engerix-B vaccine, is available ( Table 7.2 ).
| Vaccine | Dose | Schedule | Efficacy c | Adverse effects |
|---|---|---|---|---|
| Hepatitis B | ||||
| Engerix-B (GSK) | Birth to 19 yrs: 0.5 mL IM ≥20 yrs: 1 mL IM a , b | 3 doses (0, 1, and 6 mos) | 88%–96% | Injection-site soreness (22%) and fatigue (14%) |
| Recombivax HB (Merck) | Birth to 19 yrs: 0.5 mL IM ≥20 yrs: 1 mL IM a , b | 3 doses (0, 1, and 6 mos) | 96% | Injection-site reactions (18%) and systemic adverse reactions (15%) |
| Heplisav-B (Dynavax) | ≥18 yrs: 0.5 mL IM | 2 doses (0 and 1 mo) | 96% | Injection-site pain (23%–39%), fatigue (11%–17%), and headache (8%–17%) |
| Hepatitis A/B | ||||
| Twinrix (GSK) | ≥18 yrs: 0.5 mL IM | 3 doses (0, 1, and 6 mos) | 98.5% | Injection-site soreness (35%–41%), redness (8%–11%), headache (13%–22%), and fatigue (11%–14%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree