14 Oncoplastic Surgical Techniques for the Partial Mastectomy
Introduction to Oncoplastic Surgery
For breast conservation to be efficacious, the surgeon needs (1) to obtain complete excision of the cancer with adequate surgical margin width and (2) to achieve a surgical result that maintains the breast’s shape and appearance over time.1 For larger cancers, it can be technically challenging to simultaneously address both of these goals in the same operation. Simple flap advancement “mastopexy” techniques developed by plastic surgeons for breast reduction can reshape the breast immediately after larger breast cancer resections, while minimizing deformities that can develop during radiation therapy.2 This novel approach was first referred to as “oncoplastic surgery” by W. Audretsch3 in 1994.
By contrast, small- or intermediate-sized cancers can generally be managed nicely using simple oncoplastic techniques that facilitate wide excision of the cancer and preserve the shape and appearance of the breast. In oncoplastic surgery, by advancing locally available fibroglandular tissue along the chest wall, the defect created by partial mastectomy is closed with a breast “fibroglandular flap,” called mastopexy closure. Multiple technically simple techniques have been described, all following these same surgical principles. These basic oncoplastic operations are easily taught to and used by surgeons with experience in breast surgery. In a review of 84 women who underwent partial mastectomy and radiation therapy, Kronowitz and colleagues4 showed that immediate repair of partialmastectomy defects with local tissues results in a lower risk of complications (23% to 67%) and better aesthetic outcomes (57% to 33%) than that with a latissimus dorsi flap. The latissimus dorsi flap is preferred for delayed reconstruction, and the TRAM flap is best reserved for total mastectomy defects in case of local recurrence.5 In this chapter, we describe a collection of oncoplastic procedures that apply local breast tissue flap advancement.
Anatomic Distribution of Cancer in the Breast
The design of the traditional lumpectomy commonly used in the United States originated mainly from the NSABP (National Surgical Adjuvant Breast and Bowel Project) B-06 study initiated in 1976 under the direction of Dr. Bernard Fisher. This large trial helped establish the equivalency of breast-conserving surgery and mastectomy in terms of survival.6 In the traditional lumpectomy, a curvilinear incision is placed directly over the cancer, no skin island is removed, the cancer is excised in a minimalist fashion with the intention of obtaining negative but not necessarily wide margins, and the skin is closed without any formal attempt to obliterate the lumpectomy pocket. In the B-06 trial, there was no predefined amount of normal tissue to be removed around the tumor; a tumor-free margin of 1 mm—or even less—was considered adequate. Fisher6 hypothesized that breast cancer is fundamentally a systemic disease, and in the course of doing so, he minimized the issues surrounding local control of disease.
With the use of more limited resections, a higher risk of local recurrence would be expected. Multiple studies have confirmed this hypothesis. In the Milan trial, for example, 705 patients were randomized to receive lumpectomy (excision with narrow margin) or quadrantectomy (excision of surrounding normal tissue of 2 to 3 cm). Even though the rates of distant metastases and survival were no different between the two groups, the rate of local recurrence at 5 years was much higher in the lumpectomy group (7% compared with 2.2%).7 However, since lumpectomy was developed for breast conservation, little thought was given to understanding the anatomic orientation of cancers in the breast. For good oncoplastic surgical technique, understanding of common patterns of cancer distribution is mandatory.8
Segmental Anatomy of the Breast
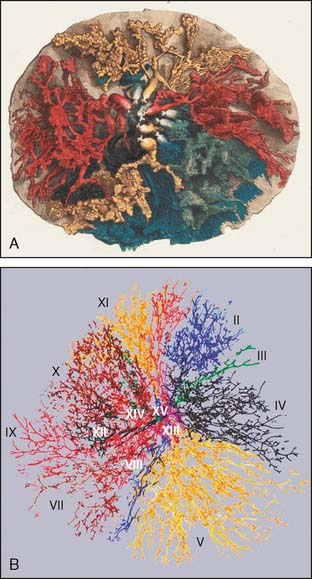
In 1840, English surgeon and anatomist Sir Astley Paston Cooper (1768–1841) published his book, On the Anatomy of the Breast.9 Cooper, who became the namesake for the breast’s suspensory ligaments, provided technically precise descriptions of breast anatomy that remain surprisingly accurate even by modern standards (Fig. 14-1A).
The number of ductal orifices and ductal segments in the breast has been a subject of controversy. The number of central ducts terminating as ductal orifices at the nipple has variably been quoted as 15 to 20 and 15 to 25 or more, although these figures have not been based on documented anatomic studies.10 To the contrary, Cooper reported, “The greatest number of lactiferous tubes I have been able to inject has been twelve, and more frequently from seven to ten.” Cooper suggested that the radiation of one of the mammary tubes commonly occupied one-sixth to one-fifth of the circumference of the breast, findings that have been verified by modern investigators. Dr. Susan Love used a combination of in vivo and ex vivo analyses to show that more than 90% of all nipples examined contained only five to nine ductal orifices, generally arranged as a central group and a peripheral group.11
Another set of investigators, Going and Moffat from the University of Glasgow, used 2-mm serial subgross sections to generate three-dimensional computer modeling images of the ductal tree in an autopsied breast.10 They described three populations of ducts: (1) seven major ducts that maintained a wide lumen up to the skin surface; (2) 20 ducts that tapered to a minute lumen at their origin in the vicinity of skin appendages of the nipple; and (3) a minor duct population arising at the base of the papilla. Going and Moffat found one dominant branching duct that drained as much as 23% of the total breast’s volume and observed that the largest six systems drain 75% of the total breast’s volume in most cases (Fig. 14-1B). These observations all suggest that the number of major ductal systems is fewer than 10 and that the anatomy is variable, with ductal segments of differing sizes and distributions throughout the breast. Accordingly, a major ductal system can represent either a very small percentage of the breast’s glandular tree or up to one fourth of the total breast volume.
In some regions of the breast, the ductal branches extend full thickness from skin to chest wall, but in other regions, the ductal branches are layered from anterior to posterior. Cooper noticed, “On the sternal (medial) and clavicular (superior) aspect of the breast, a single duct radiates to the margin; but upon the axillary (lateral) and abdominal (inferior) aspects, two or three ducts ramify to the circumference of the gland, so that two or three ducts are placed upon each other.”9 Moffat and Going12 also found that some of the ducts pass back from the nipple for a much longer distance before ramifying in the deep part of the breast. Similarly, Ohtake13 also found several ductal systems overlapping one another in the same region of the breast.
Not all ducts pass radially from the nipple to the periphery of the breast. Cooper noted one group of central ducts which branched directly toward the chest wall without extensive arborization into the periphery of the breast. In another retrospective study of 1312 archival ductograms collected by Sartorius, the ducts did not all extend in a radial fashion from the nipple; rather, some traveled directly back from the nipple toward the chest wall, with the peripheral ducts draped over the central ducts in a radial fashion.11 For a cancer found centrally, therefore, it is possible that only a central duct is involved.
Cooper9 stated that, with rare exception, the ducts ramify but do not communicate with each other. Cooper had observed a single instance of two intercommunicating ductal branches among 200 cases, which he described as a rare deviation from a general law. Ductal anastomoses were found in 4 of 16 ductal systems in Ohtake’s computer model. However, similar anastomoses could not be identified in other studies.10–12,14 The communication between different ductal systems, previously suspected to be the cause of multicentricity in DCIS, is a rare event, if it exists at all.
Cooper was the first to observe that the breast’s arterial supply comes from multiple sources, with plentiful anastomotic interconnections. He noted, “The most common supply of arterial blood in the human subject is derived from the axillary and internal mammary arteries.…The posterior or axillary branch may be seen to form a circle around the nipple, and a network with frequent communications upon the surface of the breast.”9 The well-collateralized vasculature of the breast has made it possible to move and remodel its glandular tissue safely without resultant tissue ischemia or loss.
Common Patterns of Cancer Distribution
DCIS and lobular carcinoma in situ (LCIS) generally arise in terminal ductal-lobular units or in the lobules themselves.15 In a study of 119 mastectomy specimens, Holland16 found that the distribution of DCIS is typically segmental within one ductal system. DCIS was often multifocal and on histologic examination was found to contain many small tumor foci within a single ductal branch. In poorly differentiated DCIS, 90% showed predominantly continuous growth. In contrast, only 30% of well-differentiated DCIS demonstrated a continuous distribution. Eight percent (5 of 60) showed a discontinuous (multifocal) growth with a gap wider then 10 mm.
In cases of poorly differentiated DCIS, preoperative margin assessment may be more reliable than with well-differentiated DCIS, because the microcalcifications associated with higher-grade lesions often appear mammographically as linear, branching, or coarse granular calcifications. This finding generally corresponds well to the amorphous calcifications seen at histology. In contrast, the microcalcifications associated with well-differentiated DCIS are typically seen on imaging studies as multiple clusters of fine granular microcalcifications, which correspond to the clusters of laminated, crystalline calcifications found at histology. The fine granular microcalcifications seen with lower-grade disease, which at times are subtle, may result in an underestimation of the extent of disease on mammogram.17
Finally, in terms of invasive breast cancers, most lesions have been shown to have an associated component of in situ carcinoma.18 Holland14 found that 12% of the patients had prominent DCIS (defined as six or more microscopic low-power fields of intraductal carcinoma) beyond 2 cm of the edge of the invasive tumor. This common finding should be taken into consideration when planning one’s operative conduct.
By correlating three-dimensional MRI with precise histopathologic maps, Amano and colleagues19 classified the distribution of breast cancer into three patterns: (1) localized (n = 30), (2) segmentally extended (n = 19), and (3) irregularly extended (n = 5). The segmentally extended pattern showed diffuse enhancement along duct-lobular segments, forming a cone shape. Histologically, DCIS was distributed segmentally. Mai and colleagues20 studied the pattern of distribution of intraductal and infiltrating ductal carcinoma in 30 mastectomy specimens with infiltrating carcinoma less than 3 cm in diameter. Intraductal carcinomas showed a “fanned out” pattern of distribution and frequently extended toward the nipple (with involvement of the nipple or subareolar tissue in 7 of 30 cases). In their study of 62 lumpectomy specimens for DCIS, the margins were identified as proximal (closest to the nipple), distal (farthest from the nipple), or peripheral (neither proximal nor distal). They found that positive or close margins were associated with proximal lesions in 6 cases and peripheral lesions in 13 cases, whereas none was found with distal cancer.21 These findings demonstrate that invasive ductal carcinoma more commonly arises at the periphery rather than in the center of the originating DCIS bed. Thus, cancer resection intending only to remove palpable invasive carcinoma often produces inadequate surgical margins for noninvasive cancer and leaves residual tumor cells in the remaining breast.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree