d. Other agents. Corticosteroids may be effective in controlling hypercalcemia due to sarcoidosis or other granulomatous diseases. A dose of 20 to 40 mg/day prednisone orally or its equivalent can be given to control hypercalcemia from excess ingestion of vitamin D or endogenous production of calcitrol. In patients with malignancies producing 1,25dihydroxyvitamin D such as myeloma or lymphomas, IV steroids can be used alone or in combination with bisphosphonates before definitive therapy of the underlying malignancy. Steroids are not effective in the treatment of hypercalcemia in patients with solid tumors. Hemodialysis is usually reserved for severe cases of hypercalcemia not responding to recommended treatments. Several new inhibitors of bone resorption such as osteoprotegerin (an antagonist of RANKL receptor), monoclonal antibodies directed against RANKL, monoclonal antibodies neutralizing PTHrP, and 22-oxacalcitriol are being studied currently.
B. Tumor lysis syndrome
1. Pathophysiology. The tumor lysis syndrome (TLS) results from excessive tumor breakdown either spontaneously or during therapy, leading to sudden and large amount of potassium, phosphates, and nucleic acids into systemic circulation. Catabolism of nucleic acid to uric acid causes hyperuricemia and other variety of metabolic abnormalities such as hyperkalemia, hyperphosphatemia, secondary hypocalcaemia, and acute kidney injury. The TLS occurs most frequently in patients with tumors having high growth rate and substantial systemic tumor burden that are very sensitive to chemotherapy and radiotherapy such as leukemia’s (ALL) and high-grade lymphomas (Burkitts). TLS is rarely encountered in patients with epithelial malignancies.
2. Signs and symptoms. The onset of TLS can be before the initiation of cytotoxic therapy, but is often within 12 to 72 hours after administration of cytotoxic therapy and/or radiation therapy (RT). A high level of suspicion is required because TLS symptoms can be very nonspecific and symptoms are often a result of associated electrolyte imbalances. The patients may present with nausea, vomiting, diarrhea, anorexia, congestive heart failure, cardiac arrhythmias, seizures, tetany, syncope, and possibly sudden death, often due to cardiac arrest.
The most worrisome metabolic consequences of TLS are hyperkalemia, hypercalcemia, and renal failure. Acute renal failure results from precipitation of phosphate and uric acid in the renal tubules causing renal vasoconstriction decreased renal blood flow and acute kidney injury, which creates a vicious cycle, thereby resulting in further deterioration of renal function. Patients with baseline elevated levels of uric acid, phosphorus, and lactate dehydrogenase (LDH) before treatment may be at risk for TLS.
3. Classification of TLS. Laboratory TLS (LTLS) is defined as the presence of two or more of the following laboratory parameters within three days before or seven days after cytotoxic chemotherapy: (a) uric acid ≥8 mg/dL or 25% increase from baseline; (b) potassium ≥6.0 mEq/L or 25% increase from baseline; (c) phosphate level ≥4.5 mg/dL or 25% increase from baseline; and (d) calcium ≤7 mg/dL or 25% decrease from baseline. Clinical TLS (CTLS) is defined as the presence of laboratory TLS plus at least one clinical complication such as renal failure and/or cardiac arrhythmias and/or seizures and/or sudden death (Br J Haematol 2004;127:3).
4. Management. Management of TLS involves treatment of the underlying electrolyte abnormalities along with coexisting renal failure or cardiac arrhythmias. The best approach to managing TLS is prevention. Tumor- and patient-related factors are used to calculate the risk of TLS in individual patients. High-risk patients are pretreated with isotonic saline to maintain high urine output (80 to 100 mL per hour) and are recommended to give at least single dose of Rasburicase and should also be pretreated for at least 2 days with allopurinol (600 mg/day). Uric acid levels are closely monitored and dose of Rasburicase may be repeated for persistent hyperuricemia. The dose of allopurinol should be decreased for preexisting renal insufficiency.
a. Intermediate-risk patient are also pretreated with isotonic saline to maintain high urine output and pretreatment allopurinol if the baseline uric acid levels are <8 mg/dL. If the uric acid levels are ≥8 mg/dL, Rasburicase should also be given.
b. For low-risk patients, a wait-and-watch approach is used with hydration and close monitoring.
c. Furosemide may be given to maintain urine output and also to decrease the hyperkalemia. Urine alkalinization with either one ampoule of NaHCO3 in 0.5 N saline or two to three ampoules in D5W may be needed to maintain the urine solutes (calcium, uric acid, and oxalates) in ionic form and thereby prevent crystallization and also to help correct metabolic acidosis accompanying TLS. Blood chemistries (electrolytes, creatinine, phosphorus, calcium, and LDH) need to be checked in patients at risk every 8 to 12 hours during the first 2 to 3 days of treatment.
d. Hyperkalemia may develop rapidly, and patients at risk should have serum electrolytes checked at least every 12 hours, and more frequently if TLS develops. Mild hyperkalemia (5.5 to 6.0 mEq/L) may be treated with sodium polystyrene sulfonate (Kayexalate resin) and hydration. More severe hyperkalemia (greater than 6 mEq/L or with electrocardiogram [EKG] changes) may be treated immediately with 50 mL of 50% glucose solution with 15 U of regular insulin, i.v. piggyback over an hour. Indications for hemodialysis include volume overload, serum uric acid greater than 10 mg/dL, or rapidly increasing phosphorus levels and uncontrolled hyperkalemia. Renal failure caused by TLS is usually reversible, and even patients requiring hemodialysis often regain normal kidney function as the TLS subsides.
C. Syndrome of inappropriate antidiuretic hormone (SIADH)
1. Pathophysiology. SIADH is a syndrome of excessive inappropriate secretion of ADH resulting in water retention and hyponatremia. Excessive secretion of ADH causes increased urine osmolality and increased sodium loss, resulting in concentrated urine. Small-cell lung cancer (SCLC) is the most common cancer causing SIADH, with over 15% patients with SCLC developing SIADH at some point during the course of the illness. Head and neck cancers, olfactory neuroblastomas, and extrapulmonary small-cell carcinomas are some less common causes of SIADH.
2. Signs and symptoms. Patients initially present with headache and fatigue and if left uncorrected may rapidly progress to confusion, seizure, coma, and death. A low plasma osmolality with elevated urine osmolality (more than 100 mosmol/kg), urine sodium more than 40 mEq/L, low blood urea nitrogen (BUN) 10 mg/dL, serum uric acid level <4 mg/dL, FENa >1%, and normal acid–base and serum potassium levels are all suggestive of SIADH.
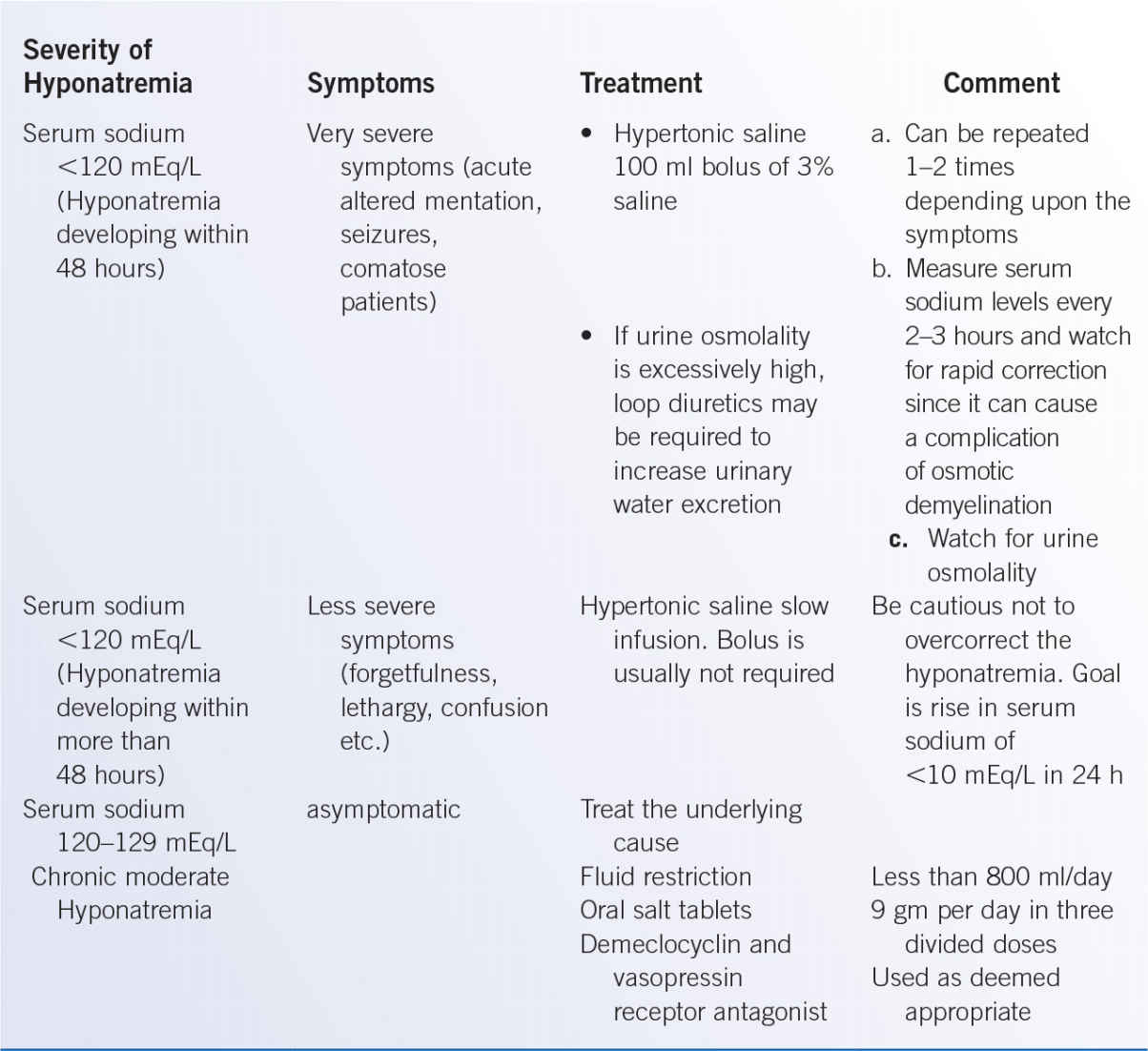
3. Management. The treatment of SIADH varies with the severity of hyponatremia and presence of symptoms. Fluid restriction is the initial step of management for patients with mild-to-moderate SIADH. In patients with acute (less than 48 hours) and severe symptomatic hyponatremia, an urgent intervention with 100 mL of hypertonic saline is given as bolus and can be repeated 1 to 2 times at 10 min interval depending upon the persistent of neurological symptoms. This treatment should raise the serum Na concentration by approximately 1.5 to 2.0 mEq/L. For patients with serum sodium <120 mEq/L and less severe symptoms (more than 48 hours), the goal of correction is to increase serum sodium 1 mEq/L per hour for 3 to 4 hours. When the serum Na levels reach 120 mEq/L, then the 3% saline should be stopped and fluid restriction instituted. For patients with no symptoms or mild symptoms, initial treatment with fluid restriction with oral salt tablets is recommended (Table 38-2).
a. Maintenance therapy in previously symptomatic patients is to maintain fluid restriction to less than 800 mL/day with monitoring of serum sodium levels to >130 mEq/L (Table 38-3).
b. For chronic SIADH, treatment options include demeclocycline (300 to 600 mg daily) and vasopressin receptor antagonists such as tolvaptan (15 to 60 mg oral daily) or conivaptan (20 to 40 mg intravenously daily) (J Endocrinol Metab 2013;98:1321).
Maintenance Treatment in SIADH |
Treatment | Dose | Comment |
Fluid restriction | Intake less than 800 mL/d | Do not restrict fluids in patients with SIADH from subarachnoid hemorrhage |
Oral salt | 9 g/d in three divided doses | May need to increase the dose to increase urine volume |