Figs. 8.1 and 8.2
A superior aesthetic result from an implant-supported prosthesis rivalling any potential result achievable with autogenous tissue transfer
Head and neck patients offer a unique set of challenges and relative contraindications to implant placement that include poor systemic health and nutritional status, poorly controlled metabolic diseases, ongoing or recent chemotherapy and/or radiotherapy and continued smoking.
Fundamentals of Maxillofacial Implantology
Implants have been employed in oral prosthodontic rehabilitation since the 1980s [1]. The history of dental implantology goes back much further to implantation of human teeth supported by gold strips by the Etruscans as early as 630 BC [2]. Much of what we know about implantology today hinges on work done by Branemark in the 1960s and the key observation of osseointegration of titanium fixtures to bone as a foundation for the science implantology has become today [3].
Successful implant placement is based on achieving osseointegration through good primary stability followed by secondary stability. Osseointegration is defined by Zarb and Albrektsson as “direct structural and functional connection between ordered, living bone and the surface of a load-carrying implant” [4]. Primary stability is the initial stability at the time of placement by mechanical engagement of an implant with the surrounding bone and is dependent upon bone density and implant design. Tapered implants, thinner drills and omitting pre-tapping (where the bone is grooved so the implant “screws” into the bone) are all strategies that can enhance primary stability [1]. Secondary stability is bone regeneration and remodelling to determine the bone implant contact and final osseointegration.
One can distinguish two different forms of osteogenesis in implant osseointegration: contact osteogenesis and distance osteogenesis [5]. The former is bone forming directly on the implant surface whilst in the latter instance bone is formed from pre-existing bone surfaces migrating towards the implant. Surface-treated implants with roughened surfaces tend to show more contact osteogenesis and better short- and long-term outcomes, and the recent trend in implantology has been towards favouring these over machined, smooth-surface implants in view of these superior outcomes [6].
Criteria for successful implant integration have been described by Zarb and Albrektsson [4] as:
- 1.
The implant allows for placement of planned functional and aesthetic prostheses.
- 2.
The absence of pain, discomfort, altered sensation or infection.
- 3.
Individual implants are immobile.
- 4.
Mean vertical bone loss less than 0.2 mm/annum following the first year of function.
Implant-Supported Obturators and Removable Prosthodontic Devices
Obturators represent an attractive solution in terms of rehabilitating patients with partial and total maxillectomy defects. They allow for minimal surgical morbidity, obviating the need for free flap harvest and reconstruction and lengthy surgery whilst yielding good aesthetic and functional results as well as allowing for regular easy inspection of the ablative site to enable early detection of recurrence. However, patients with partial and total maxillectomy defects may present significant challenges that need to be addressed [7]:
- 1.
Masticatory problems in partially dentate/edentulous patients
- 2.
Hypernasality due to oro-nasal/oro-antral communication and speech defects
- 3.
Difficulty in retaining removable prostheses
- 4.
Facial disfigurement due to loss of maxillary support for overlying soft tissues
- 5.
Dysphagia due to loss of velopharyngeal competence and safe swallow
Where a decision is made to provide an implant-assisted obturator, implants are usually placed in the premaxilla and tuberosity regions, as these areas of the maxilla often represent the best quality and volume of bone available [8]. A surgical guide can be used and pre-planned using software planning packages or stents fabricated on plaster models.
As highlighted by Roumanas and colleagues [8], we recognize that uniting implants with a precision-fitted bar directs occlusal forces along the axis of the implants to generate more favourable loading patterns and optimize survival of the implants.
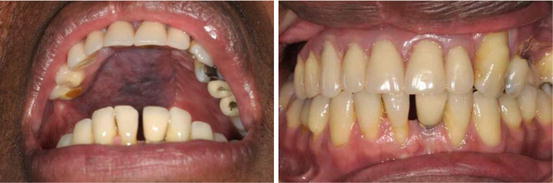
In the absence of adequate bone in the maxilla, zygomatic implants can represent a useful alternative solution. In recent years we have placed less of these as our tendency in such situations in Birmingham is to place composite free flaps to provide adequate bone stock for standard implant placement (Figs. 8.3 and 8.4).
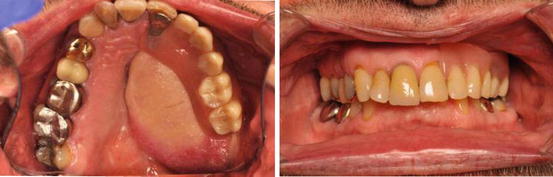

Figs. 8.3 and 8.4
An example of a removable implant-supported prosthesis on a osseocutaneous composite radial forearm free flap
We recognize the need to link implants again with bar devices, as we have seen higher failure rates when zygomatic implants are placed in isolation. In our own series of 53 implants in 42 sites, we have had six (9.5 %) failures [9]. Other authors have demonstrated similar outcomes for use of zygomatic implants for maxillary and midface defects [10, 11].
Implant Placement in Composite Flaps
Composite flap (bone with skin or muscle) reconstructions of the oral cavity aim to achieve adequate width and height of bone to replace horizontal and vertical deficiencies following ablative surgery. Selection of free flaps should take into account their ability to support implants as well as adequate pedicle length to avoid where possible the need for inter-positional vein grafts, sufficient bone to restore facial contour whilst always being mindful of the need to minimize donor site morbidity.
All flaps are not equal when it comes to considering their ability to support implants. Moscoso et al. have described different free flaps as having a relative implantability index, whereby the iliac crest is the most consistently implantable donor site, followed by the scapula, fibula and radius (83 %, 78 %, 67 % and 21 %, respectively) [12]. Free flap selection in orofacial reconstruction should take into account the potential volume of bone harvested to “replace like with like” and also provide sufficient bone stock to support implants as well as sufficient bone quality as described by Lekholm et al [13]. Implant survival rates of 93 % have been reported for fibula flaps in the literature, with a 98 % implant-supported prosthesis success rate, making outcomes predictable and safe [14, 15] (Figs. 8.5, 8.6 and 8.7).
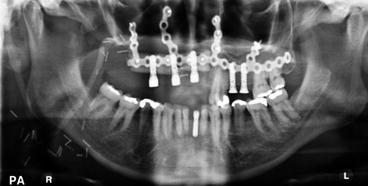

Fig. 8.5
Orthopantomogram (OPG) showing fibula flap used for reconstruction of partial maxillectomy defect incorporating pre-bent custom reconstruction plate and osseointegrated implants
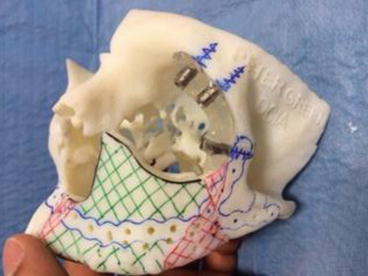
We regularly employ computer-assisted design (CAD) in our more complex reconstructions. Using DICOM data from 3D computed tomograms (CT) of donor and recipient sites for composite flaps we construct stereolithographic (STL) models. The Object 3D printer we use provides highly accurate rendering at 27 μm per slice. Such STL models enable the manufacture of custom reconstruction plates (laser-welded titanium 2.0 mm locking plates), which are pre-fabricated in the laboratory, saving valuable intra-operative time and ensuring accurate results. STL models also enable the fabrication of cutting guides for donor sites such as the deep circumflex iliac artery (DCIA) free flap, fibula and scapula [16, 17] (Figs. 8.8, 8.9, 8.9, 8.10 and 8.11).
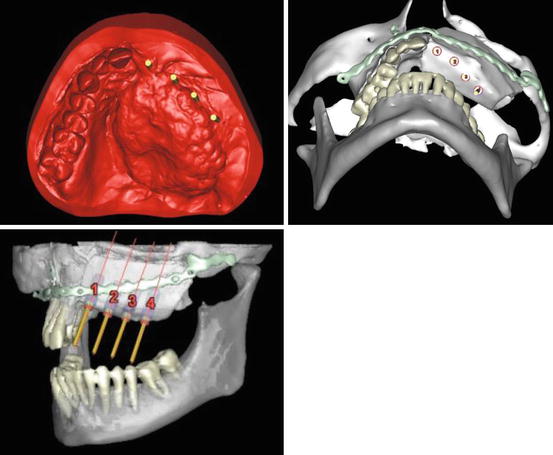

Figs. 8.8–8.10
Computer-assisted design (CAD) planning for a deep circumflex iliac artery (DCIA) free flap reconstruction of partial maxillectomy defect

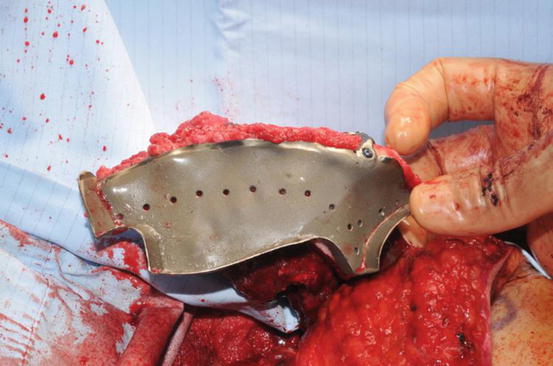
Fig. 8.11
A computer-designed custom cutting stent for a bespoke deep circumflex iliac artery (DCIA) free flap with holes for a pre-bent custom reconstruction plate
Such technology can equally be employed further down the line for custom made implant stents in conjunction with our restorative dentistry colleagues and maxillofacial prosthetists. The option exists, however, for implant placement at the time of ablative surgery using suitable custom stents, and this is well described in the literature [18, 19]. Jackson et al [14] have demonstrated no difference in placement at either the initial surgery or a separate second stage approach and this is our experience also (Fig. 8.12).