Neoplastic
Non-neoplastic
Ductal adenocarcinoma
Lymphoepithelial cyst
Mucinous cystadenocarcinoma
Congential cyst
Pancreatoblastoma
Mucinous non-neoplastic cyst
Neuroendocrine tumor
Enterogeneous cyst
Solid pseudopapillary tumor
Retention cyst
Serous cystadenoma
Duodenal cyst
Mucinous cystadenoma
Endometrial cyst
Intraductal papillary mucinous neoplasm
Pseudocyst
Sarcoma
Parasitic cyst
Lymphoma
Autoimmune pancreatitis
Metastatic lesion
Splenule
Dermoid cyst
Fat necrosis
Hamartoma
Acinar cell cystadenoma
von Hippel-Lindau cystic neoplasm
Cholangiocarcinoma
Gastrointestinal stromal tumor
The diagnosis of incidentaloma may be a great opportunity for eradication of a malignancy or precursor even at high volume centers, mortality and morbidity following pancreatic surgery is 3 and 50 %, respectively [3]. In addition, surgical intervention and management of these patients carries a financial burden [7].
In the context of an incidentaloma, the benefit of early detention with subsequent aggressive surgical resection versus the morbidity and mortality is a delicate balance for both patient and the health care institution. The following review will discuss the risk of malignancy, etiology of, and the current recommendations for management of pancreatic incidentalomas, particularly cystic lesions. Excluded from this review are lesions of the pancreas that are symptomatic including sequelae of acute, autoimmune, or chronic pancreatitis (i.e. pancreatic pseudocyst) and advanced carcinoma (primary or metastatic).
The Threat: Risk of Malignancy
The concern of any lesion in the pancreas is malignancy. Reports indicate that up to 60 % of lesions of the pancreas harbor malignant or pre-malignant diagnoses [8]. Early detection and resection leads to favorable survival for those with malignancy [9]. In one report, 5-year survival was 64 % for incidental lesions as compared to 47 % for non-incidental lesions with a median survival of 145 versus 46 months. Disease-specific survival for adenocarcinoma was improved by 3 months for incidental lesions compared to non-incidental lesions [10].
Multiple high volume pancreatic groups have presented their experience with pancreatic incidentalomas. Vollmer et al. presented a 5-year experience showing 17 % of incidentalomas were invasive cancer [11]. Lahat et al. showed that 34 % of patients with pancreatic incidentalomas harbored malignancy [10]. Importantly, this rate was half of those with symptomatic lesions. But, 23 % of the incidental group had the diagnosis of intraductal papillary mucinous neoplasm (IPMN), a pre-malignant lesion.
In these reports, invasive lesions were adenocarcinoma of the pancreas. However, neuroendocrine tumors, cholangiocarcinoma, and metastatic lesions can also be seen in a minority of cases [10, 12]. The operations required to extirpate these lesions include pancreaticoduodenectomy (approx. 30 % of the time), central (8 %) and distal pancreatectomy (30 %), total pancreatectomy (4 %), and enucleation (2–22 %) [12]. Features of the each common incidentaloma are summarized in Table 2. Important to note, lesions of the pancreas that are symptomatic have an indication for surgery in surgically fit patients. Extensive laboratory and invasive workup is not necessary for these patients. However, in those without symptoms better definition of the disease is required to tailor intervention or surveillance.
Table 2
Summary of clinical feature of common incidentalomas
Entity | Serum | Cystic contents | Ultrasound | ERCP | CT/MRI |
|---|---|---|---|---|---|
IPMN | Normal | High CEA | Mural nodules, ductal dilation | Communicates with duct, ductal dilation | Mural nodules, ductal dilation |
MCN | Normal | High CEA, CA 19-9, low amylase | Round, smooth, large cyst | No ductal involvement | Round, well circumscribed, septations, macrocystic |
SCN | Normal | Low CEA, CA 19-9, amylase | Septations, calcifications | No ductal involvement | Septations, honeycomb, central scar, hypervascular |
Pseudocyst | Normal | High amylase, low CEA, CA 19-9 | Anechoic, smooth, round | Ductal communication possible | Round, fluid filled, smooth, unilocular |
Lymphoepithelial Cyst | Normal | High CEA, CA 19-9 | Hypoechoic, internal debris | No ductal involvement | Low attenuation, internal keratin |
PNET | High chromogranin, GI hormone if functioning | Low or normal CEA, CA 19-9 | Smooth, round, loculations variable | Normal duct | Hyperattenuation |
SPT | Normal | NA | Hypoechoic | Ductal involvement variable | Cystic degeneration, solid and cystic |
Cystic Incidentalomas
Most of pancreatic incidental lesions (52 %) are cystic rather than solid [11]. Cystic lesions can be classified into congenital (serous and multicystic syndromes), inflammatory (pseudocysts), and neoplastic (serous cystadenoma/SCN and mucinous cystic neoplasms/MCN, and intraductal papillary mucinous neoplasms (IPMN, main-duct and branched-duct)). Typically inflammatory and main-duct IPMN lesions are symptomatic. The cystic neoplasms represent 90 % of incidental cystic lesions of the pancreas [13]. Patients present with CT of the chest, abdomen, or pelvis that lacks the resolution to adequately define the pancreatic lesion and its resectability. Following a comprehensive history and physical, repeat CT or MRI with a pancreatic protocol is optimal to better define the lesion and predict its natural history.
SCNs have a well-demarcated wall with multiple septated cysts within its mass described as a honey-comb lesion. Most SCNs are found in the pancreatic head. Symptoms relate to mass effect: gastric outlet obstruction, early satiety, and jaundice. Each cyst is <2 cm filled with clear fluid void of mucin (carcinoembryonic antigen/CEA). SCNs are typically single and located in the pancreatic parenchyma but can become quite large (up to 25 cm). Importantly, these lesions are considered benign but as they enlarge can cause symptoms. Once symptomatic, surgically fit patients should undergo an appropriate resection. If these lesions have mural nodularity, however, the diagnosis of SCN should be questioned. Further workup or early resection is warranted as nodularity is a worrisome finding for malignancy. Only 25 cases of a malignancy have been reported [14]. The long-term survival for these lesions appears to be very favorable following resection.
MCNs are the most common cystic lesion of the pancreas [15]. They do have malignant potential. Ten to 50 % of MCNs harbor malignancy. These lesions, as their name implies, produce mucin from their mucin-producing epithelial wall. MCNs do not communicate with the pancreatic duct. Histologically, the cyst wall is mucin-rich with ovarian-like stoma with estrogen and progesterone receptors in most cases. Most lesions are found in the body and tail but can, though less commonly, be in the pancreatic head and uncinate. Usually these present as a single cyst with fine septations with a rim of calcification and considered macrocytic in contrast to SCNs, which are microcytic. Following appropriate abdominal imaging, endoscopic ultrasound (EUS) is recommended for biopsy and cyst fluid analysis. A cyst fluid level of CEA>192 ng/mL (which implies a mucinous lesion) with a low amylase has an 80 % diagnostic accuracy for MCN. In contrast, a pseudocyst has a high amylase and low CEA level [16]. Once the diagnosis is made, resection is recommended for all surgically fit patients [17]. Prognosis is dependent on pathology: benign, borderline, or malignant. In the setting of malignancy, 5-year survival following resection is 57 % [18]. Survival is better than those with pancreatic adenocarcinoma.
Much like MCNs, IPMN can present anywhere in the spectrum of tumor progression from a benign adenoma, to borderline or dysplastic, carcinoma in situ (PanIN), and invasive carcinoma. IPMNs are described based on the pancreatic duct morphology in relation to the cyst. Branch-duct IPMN do not involve the main duct of Wirsung. Lesions affecting the main duct are labeled main-duct IPMN. Side-branch IPMNs that extend to the main duct are called mixed-type IPMN. These lesions are characterized by dilation of the pancreatic duct. All these subtypes are pre-malignant and invasive cancer is found in 20–50 % of resected pancreatic specimens [19]. Features that are worrisome for malignant degeneration are cysts >3 cm, main duct diameter of 1 cm, mural nodules, size, main-duct disease, cyst wall thickening, and elevated CA 19-9. Likewise, symptoms such as jaundice are associated with an increased likelihood of a malignancy.
International, contemporary management of mucinous neoplasms of the pancreas is summarized by Tanaka et al. in 2012 [17]. These guidelines are a revision of the 2006 consensus statement, or Sendai Criteria, and highlight advances in endoscopy, CT, MRI, as well as elucidation of the natural history of these diseases at high volume centers. We strongly recommend the reader to this consensus statement. The working group clearly defines high-risk stigmata of malignancy and “worrisome” features aiding in the management and follow up for these lesions. The algorithmic approach given by this group is displayed in Fig. 1.
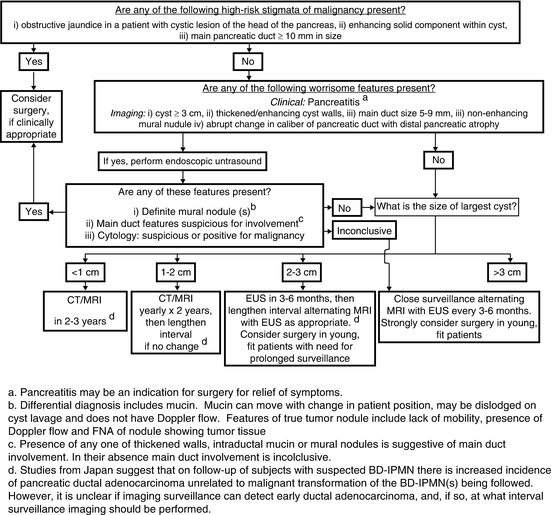

Fig. 1 Algorithim for evaluating and managing cystic neoplams of the pancreas as defined by the International consensus guidelines 2012 [17]
Another type of incidentaloma are lymphoepithelial cysts . They are rare compared to other etiologies of incidentalomas of the pancreas. They are cysts composed of a mature keratinizing squamous epithelial surrounded by lymphoid tissue. They are born from ectopic pancreatic tissue arising in a lymph node that undergoes metaplasia. In contrast to other cystic lesions of the pancreas, most are found in men. They can have a high CA 19-9 and mucin level confusing their diagnosis with IPMN. They are well demarcated with low attenuation by CT and are hypoechoic with internal debris by sonography. The use of MRI differentiates the internal keratin debris of the cyst with high T1 and low T2 signals. Characteristic MRI findings, no ductal dilatation, and biopsy help differentiate this benign lesion from more ominous entities. Resection is not warranted unless severe symptoms occur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree