Fig. 1
Interaction between different components and their overlap. The size of the circles is not an indication of the proportion but merely to high light the complex overlap
Given this complex interaction it is very important to correctly identify men in the clinical setting in order to determine who will benefit from medical or surgical management.
BOO is a urodynamic diagnosis based on observation of increased detrusor pressure and reduced urinary flow rate during voiding [3]. Although urodynamic diagnosis, with use of pressure transducers, is the gold standard, it is an invasive and time-consuming investigation. There is a constant drive to diagnose BOO in the clinical setting with the aid of simple and less invasive methods of investigation. Over the years ultrasonography has become a useful tool in the armament of the urologist to help in the diagnosis of this common problem. Assessment of many components of the prostate and bladder using transabdominal and transrectal approaches may help in the diagnosis of BOO and this is discussed in detail.
Male stress incontinence is often iatrogenic and can occur following surgery on the prostate for benign disease (e.g., by holmium enucleation of the prostate or transurethral resection of the prostate, TURP) or following radical surgery on the prostate for prostate cancer (radical prostatectomy, RP). The latter can be performed by open surgery, laparoscopically assisted, or robotically assisted. Regardless of modality, each is associated with a degree of stress incontinence with rates varying based on definition, surgical expertise, and volume.
Prostate Volume
Although the most accurate means of assessing prostate volume (PV) is via the transrectal approach, with measurement of the height, width, and length of the prostate, most modern US machines can measure PV in the transverse view using the preset ellipsoid formula [4]. Yuen et al. studied the correlation between transrectal and transabdominal measurement of prostate volume in 22 patients under general anesthesia prior to TURP. They showed that the transabdominal ultrasound measurement of PV correlated well with the transrectal measurement of the same parameter when the bladder volume is less than 400 ml [5]. Although PV is also a useful index in the evaluation of BOO, it alone is not sufficiently accurate to diagnose BOO. Indeed, as described earlier, LUTS and BOO can exist in the absence of BPE. There is some evidence to suggest that the size of the prostate may be related to the severity of obstruction and progression of BPE. A study of f 2,115 men with lower urinary tract symptoms between the ages of 40 and 79 years with a 4 year follow-up revealed that men with PV of more than 30 g were threefold more likely to develop urinary retention [6]. A videourodynamics study (VUDS) of 324 consecutive men with LUTS by Kuo revealed that 65 % of men had evidence of BOO, and PV of 40 ml or more was associated with BOO with a sensitivity of approximately 95 % [7]. Conversely, Hirayama et al. found that in a study of 36 men with PV less than 20 ml, 60 % had evidence of obstruction on pressure flow studies [8]. Presence of median lobe or bladder neck stricture (despite a small prostate volume) may in part account for the presence of BOO in this group of patients. In summary, prostate volume alone is not sufficiently accurate to exclude presence of BOO, however larger prostates are more likely to be associated with BOO.
Intravesical Prostatic Protrusions
Another parameter that can help with diagnosis of BOO is intravesical prostatic protrusions (IPP), as measured by the length of the prostate extending into the bladder in the sagittal plane. Volume of urine in the bladder will have a significant impact on the IPP. Yuen et al. have shown that the optimal bladder volume for the measurement of IPP is approximately 100–200 ml. Over-distension of the bladder (volume greater than 400 ml) leads to retraction of the prostate behind the symphysis pubis and inaccurate assessment of IPP. In contrast, under filling of the bladder (volume less than 100 ml) leads to over-estimation of IPP [5]. IPP can be graded as follows: Grade 1, 5 mm or less protrusion; grade 2, 5–10 mm protrusion; and grade 3, more than 10 mm protrusion as shown in Fig. 2 [4, 9]. In a prospective study of men over the age of 50, Chia et al. assessed the correlation between IP and BOO. In all, 125 patients had significant BOO, defined as a BOO index of >40. Of these men, 94 had grade 3 and 30 had grade 1–2 IPP, with a positive predictive value of 94 % and a negative predictive value of 79 % in Grade 3 IPP [9].
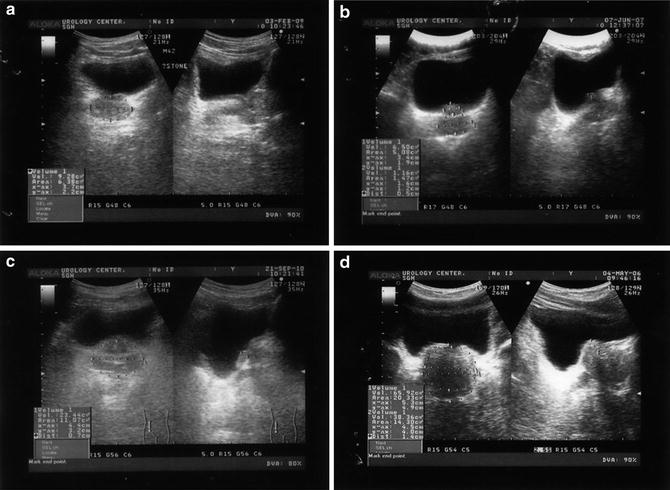

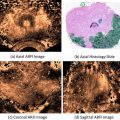
Fig. 2
(a) Normal prostate. (b) Intravesical prostatic protrusion (IPP) grade 1 prostate. (c) IPP grade 2 prostate. (d) IPP grade 3 prostate
Similarly, Kegin et al. in a review of 206 patients with BPE found patients with grade 3 IPP had a statistically lower peak flow rate (Qmax), and a significantly higher maximum detrusor pressure (Pdet.max) and BOO index (BOOI) when compared to patients with grade 1–2 IPP (p < 0.05 during urodynamic testing). The correlation coefficient (Spearman’s rho) between IPP and Qmax, Pdet.max, and BOOI was −0.284, 0.252, and 0.456, respectively. They therefore concluded that IPP is a useful predictor in the assessment of patients with BOO. Patients with grade 3 IPP had more severe BOO and impaired detrusor function [10].
Lim et al. also showed that PV and IPP have a positive predictive value of 65 % and 72 %, respectively, for BOO. The Spearman rho correlation coefficients were 0.314 and 0.507 with the area under the receiver-operator characteristic curves of 0.637 and 0.772, for PV and IPP, respectively. Using a nominal regression analysis they concluded that IPP was a better indicator for BOO compared to PV [11].
Lee et al. showed that men with Grade 3 IPP who are on medical management are sevenfold more likely to progress over a mean follow-up period of 32 months [12].
Given the ease of IPP measurement it can be used as a surrogate marker in the initial assessment of men with LUTS as well risk stratification with regards to patients on conservative management with confirmed diagnosis of BOO. However, to date, this remains an experimental tool that has not entered widespread clinical practice. In a study of 111 patients with confirmed BPE, Aganovic et al. showed that grade 3 IPP was a superior indicator for BOO, as compared to ultrasound measurement of bladder wall thickness (BWT) [13]. The authors also found that flow rate and age were useful predictors of BOO.
Transition Zone Index
Current generation US machines provide far superior image quality and allow a clear distinction between different zones of the prostate (Fig. 3, 4). As BPH arises in the transition zone tissue, some researchers advocate use of transition zone (TZ) index as an indicator for BOO. Transition zone index is calculated using a TRUS probe with the following formula: TZ volume/total prostatic volume (TZV/TPV).
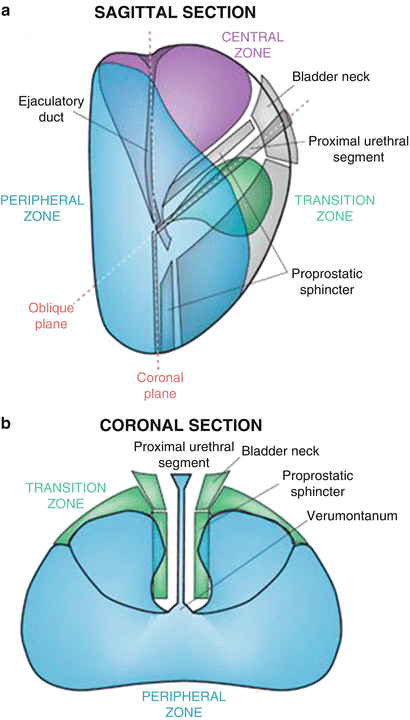
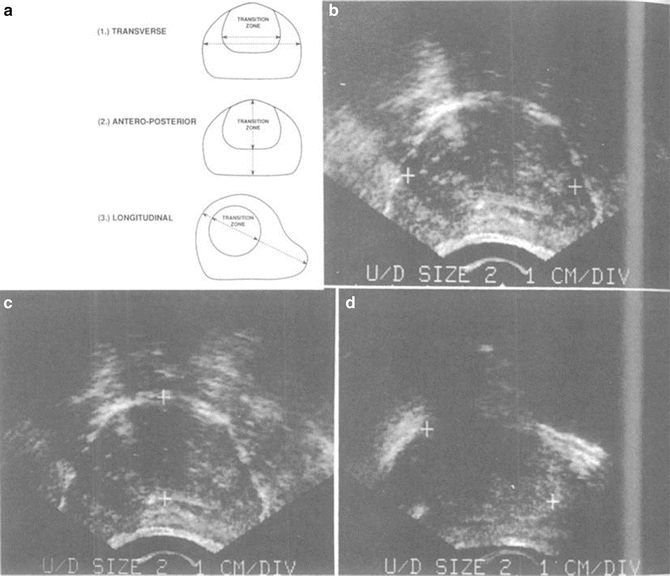

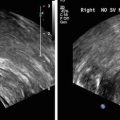
Fig. 3
(a) Sagittal (top panel) and (b) coronal (bottom panel) section of the prostate showing peripheral zone, transition zone, central zone, the verumontanum, the proximal urethral segment, as well as preprostatic sphincter, bladder neck, and ejaculatory duct

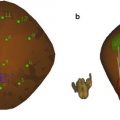
Fig. 4
(a) Measurements made of whole prostate gland and transition zone in each illustrated dimension: (b) transverse, (c) AP, and (d) longitudinal sonograms of prostate showing measurements in real time
Greene et al. demonstrated that TZ volume is significantly increased in the clinical BPH group. They found the mean size of the transition zone was 6.14 ± 3.2 g in the normal group (as defined as no US features of BPH) and 24.81 ± 14.4 g in the BPH group. The size of the transition zone increased significantly in relation to increased age of the patient [14]. Others have also demonstrated that application of TZ index can accurately identify patients with significant BOO.
Kaplan et al. conducted a prospective evaluation of 61 men with symptomatic BPH. They concluded that TZ Index demonstrated a significant correlation with American Urological Association symptom score (AUASS) (r = 0.75; p < 0.001) and peak flow rate (r = 0.71; p < 0.001). They concluded that TZ index may be valuable in the assessment of BOO [15].
Witjes et al. conducted a comprehensive study of 150 patients to establish the correlation between prostate volume, transition zone volume, transition zone index, and clinical and urodynamic investigations in patients with lower urinary tract symptoms. They concluded that there were very small differences between the correlations of total prostate volume, TZ volume, and TZ index, and clinical and urodynamics variables. The addition of TZ index was of little value in the diagnosis and management of a patient with BOO. They also concluded that TZ index and other similar parameters should not be used in isolation to guide appropriate management [16].
Resistive Index
Resistive index (RI) can be employed to assess blood flow through a target organ. Modern generation US machines are able to utilize power Doppler transrectal ultrasound (TRUS) to calculate the RI in the prostate. Power Doppler uses the amplitude of the signal to calculate the density of red blood cells irrespective of velocity or flow direction. In order to measure RI the patient is placed in the standard left lateral position, and a 5.0–8.0 MHz end-fire TRUS probe is used. Pulsed-wave spectral Doppler images are obtained with the patient in the left lateral decubitus position. On the transverse view of the prostate, pulsatile waveforms of blood flow are obtained from the capsular artery and subjected to spectral waveform analysis. The pulsatile waveforms are then stabilized for a given Doppler spectrum, and the RI is measured using on-board software (Fig. 5) [17].
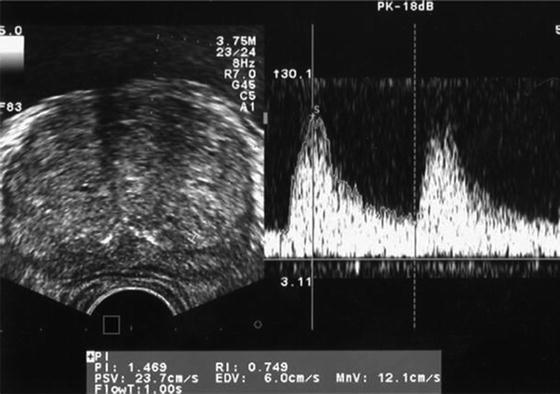

Fig. 5
Sample Doppler waveforms of blood flow at the capsular artery of the prostate. Systolic velocity and diastolic velocity are measured, and the RI is calculated using on-board software. PSV peak systolic velocity; EDV end diastolic velocity; PI pulsatility index; MnV mean velocity; FlowT flow time; RI resistive index
Tsuru et al. studied a total of 214 men aged between 48 and 86 years old with lower urinary tract symptoms. They found a significant correlation between the RI of capsular arteries and the International Prostatic Symptom Score (IPSS) (r = 0.389; p < 0.0001) and peak flow rate of uroflowmetry (r = −0.393; p < 0.0001) [18].
Kojima et al. evaluated the utility of RI in 140 patients with symptoms suggestive of BOO. Their study showed that RI was also higher in patients with infravesical obstruction than those without (0.74 ± 0.06 vs. 0.70 ± 0.05, p < 0.005). In their study, RI was significantly correlated with urodynamic parameters. They found that RI of 0.7 or more correctly identified 28 patients out of 33 (85 %) with obstruction, while 11 out of 24 patients (46 %) without obstruction had an RI less than 0.7 [19]. RI may not only be able to identify patients with BOO, but it may also help predict the patients who are likely to benefit from TURP. Haung et al. showed that RI accurately predicts the outcome of TURP. RI was more accurate in predicting effective outcome after TURP than bladder wall thickness. By combining measurements, the authors found that resistive index, detrusor wall thickness, and ultrasonic estimation of bladder weight had a combined positive predictive value of 96.3 % for successful surgical outcome [20].
It is postulated that the increase in RI noted in BOO is the result of lateral prostatic lobe enlargement compressing the prostatic capsule, with a resultant increase in RI of the capsular arteries. However, in patients with only enlargement of the median lobe as the cause of BOO, RI wound not be elevated. In addition, many conditions such as hypertension, atherosclerosis, and diabetes mellitus can lead to a decrease in tissue and vascular compliance and hence alter RI. Other limitations include the effects of drugs such as α-blockers, 5-α-reductase inhibitors, antihypertensives, and nonsteroidal antiphlogistics, which modulate the function of the capsular artery and tissue in the prostate and therefore will alter the RI. In summary, although RI can be useful in the diagnosis and prediction of surgical outcome in patients with BOO, there are many factors that can modulate the RI. As such, it is not accurate enough to replace the gold standard of urodynamics investigation of bladder function and confirmed BOO.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree