Prognostic group
# risk factorsa
Low risk
0, 1
Low intermediate
2
High intermediate
3
High
4, 5
NCCN-IPI | Score |
|---|---|
Age (years) | |
41–60 | 1 |
61–75 | 2 |
>75 | 3 |
LDH, normalized | |
>1 to ≤3 | 1 |
>3 | 2 |
Extranodal diseasea | 1 |
Performance status ≥2 | 1 |
Score | 5-y OS | 5-y PFS | ||||
|---|---|---|---|---|---|---|
NCCN-IPI | IPI | NCCN-IPI (%) | IPI (%) | NCCN-IPI (%) | IPI (%) | |
Low | 0–1 (19 %) | 0–1 (38 %) | 96 | 90 | 91 | 95 |
Low intermed. | 2–3 (42 %) | 2 (26 %) | 82 | 77 | 74 | 66 |
High intermed. | 4–5 (31 %) | 3 (22 %) | 64 | 62 | 51 | 52 |
High | >6 (8 %) | 4–5 (14 %) | 33 | 54 | 30 | 39 |
4 Treatment
Most cooperative groups tailor treatment strategies according to age and age-adapted IPI. In patients with high tumor loads, a so-called pre-phase treatment with prednisone (100 mg/d over several days up to 1 week) [26] is recommended to prevent tumor lysis syndrome and ameliorates the so-called first cycle effect, i.e., the phenomenon that side effects, in particular myelosuppression, are most pronounced after the first chemotherapy cycle. G-CSF should be given to all elderly patients and younger patients at risk for febrile neutropenias, treatment delays, and/or dose reductions.
4.1 Young Low-Risk Patients (aaIPI = 0) Without Bulky Disease
Six cycles of chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone combined with six doses of rituximab given every 21 days represent the current standard treatment for these patients [27, 28]. Results achieved with 6 × R-CHOP-21 combination in the MInT study [29] are so excellent (6-year PFS: 93 %, 6-year OS: 100 %) that there is no room for additive radiotherapy or any other treatment intensification (Fig. 1). Whether 4 cycles of R-CHOP-21 are sufficient in patients with a negative PET after 3 R-CHOP-21, as suggested by a retrospective register study of 50 patients, which was published in abstract form only already several years ago [30], remains to be confirmed. The DSHNHL is currently conducting the FLYER study which randomizes young patients with favorable prognosis (no risk factor, no bulky disease) into the MInT standard of 6 × CHOP-21 versus 4 × CHOP-21 each in combination with 6 administrations of rituximab every 3 weeks, to determine whether the number of CHOP-21 cycles can indeed be reduced in this favorable subgroup.
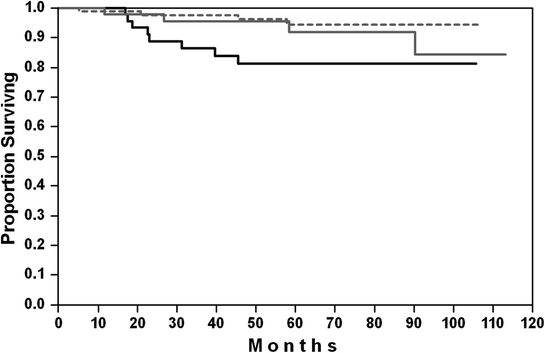

Fig. 1
Overall survival of “very favorable young patients” (no bulky disease, aaIPI = 0) in the MInT study [29]. Black solid curve CHOP-21; gray solid curve CHOEP-21; dotted line R-CHO(E)P-21
Based on the results of a phase II study with only 60 patients, a different approach to these patients is popular in North America, consisting of 3 × CHOP-21 in combination with 4 applications of rituximab followed by involved-field radiotherapy [31]. However, in contrast to the MInT results, no plateau has been observed for patients treated according to this strategy, indicating that this abbreviated chemoimmunotherapy is insufficient to eradicate the malignant clone. Interestingly, the addition of only 4 administrations of rituximab resulted in only a small improvement compared to a historical control, suggesting that this short exposure to the CD20 antibody does not exploit the full potential of this drug [31].
4.2 Young Low-Risk Patients (aaIPI = 0) with Bulky Disease and Low-Intermediate Risk (aaIPI = 1)
Bulky disease was a strong prognosticator in the MInT study [27, 29], despite the fact that patients in that study received additive radiotherapy to areas of primary bulky disease after immunochemotherapy. For CHOP-like treatment and rituximab plus radiotherapy to bulky disease, a cutoff point of 10 cm maximum tumor diameter separated two populations with a significant EFS difference, but any cutoff point of 6 cm or more separated two populations with a significant OS difference. Even though, a bulk definition as a mass with >10 cm maximal tumor diameter is applied by many cooperative groups, it must be assumed that the cutoff points for bulky disease not treated with additive radiotherapy will be even smaller than the 10 and 6 cm for EFS and OS, respectively.
Young low-intermediate risk patients (aaIPI = 1) or IPI low-risk (aaIPI = 0) patients with bulky disease have a less favorable outcome. While the overall survival of this group was ≈90 % in the MInT study, the EFS was roughly 75 % (Fig. 2), necessitating salvage treatment for about a quarter of this young population which usually consists of salvage chemotherapy followed by ASCT, with all its well-known acute and long-term toxicity. In order to improve outcome of this subgroup, the GELA NHL 03-2B study compared 8 cycles of R-CHOP-21 with the R-ACVBP-14 program (an R-CHOP-14 variant which includes consolidation with high-dose methotrexate, ifosfamide, and high-dose cytarabine). The R-ACVBP-14 program was significantly better with respect to 3-year PFS (87 % vs. 73 %; p = 0.0015) and OS (92 % vs. 84 %; p = 0.0071) in this population [32]. However, the R-ACVBP-14 program was also significantly more toxic than R-CHOP-21, with 38 % of the R-ACVBP-14 patients experiencing neutropenic fever compared to only 9 % with R-CHOP-21. Interestingly, in a (historical) comparison of this well-defined subgroup of young patients with one risk factor according to the age-adjusted IPI in the MInT and NHL 03-2B studies, 6 × R-CHOP-21 in MInT yielded considerably better results than 8 × R-CHOP-21 in the French trial and indeed appears to be as good as the more toxic R-ACVBP-14. The only plausible explanation for this paradox is that the French abandoned radiotherapy from their studies after the advent of rituximab, while the patients with bulky disease and/or extralymphatic involvement in the MInT study had received radiotherapy to the respective areas.
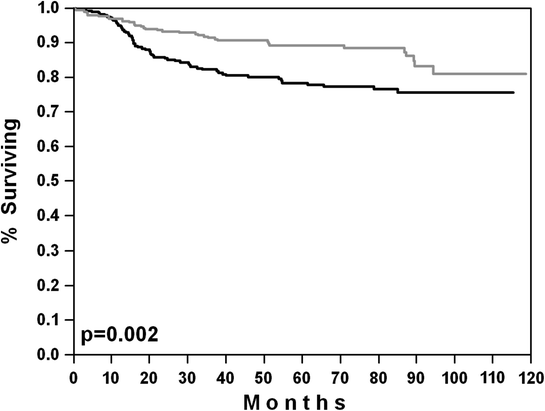

Fig. 2
Overall survival of “less favorable young patients” (aaIPI = 1; aaIPI = 0 with bulky disease) in the MInT study [29]. Black curve CHOP-like chemotherapy (n = 302); gray curve CHOP-like chemotherapy plus rituximab (n = 312)
While the comparison between MInT and NHL 03-2B was the first evidence that radiotherapy to bulky disease has still a value in the rituximab era, this assumption has recently been supported by the interim results of the randomized UNFOLDER study of the DSHNHL, which compares 6 × R-CHOP-21 with 6 × R-CHOP-14 and radiotherapy to areas of bulky and extralymphatic disease with observation in a 2 × 2 factorial design. A planned interim analysis with 285 patients revealed a highly significantly better 3-year EFS of patients randomized to radiotherapy (81 % vs. 64 %; p = 0.004); this difference was above the preset stopping rule (p = 0.008) and made the DSMC to order the early closure of the two arms of the UNFOLDER study without radiotherapy. Accordingly, the current ESMO guidelines recommend either the R-ACVBP-14 program or 6 cycles of R-CHOP-21 with radiotherapy to bulky disease for this subgroup of young DLBCL patients [28].
4.3 Primary Mediastinal B-Cell Lymphoma (PMBCL)
Most patients with PMBCL also fall into the subgroup of young patients with one aaIPI risk factor and/or bulky disease. A small study of only 48 PMBCL patients treated with dose-adjusted infusional EPOCH-R without radiotherapy made it into the New England Journal of Medicine reporting a 5-year EFS of 93 % and 5-year OS of 97 % (without showing any confidence intervals) [33]. Since PMBCL patients are recruited for the UNFOLDER study, the DSMC ordered an interim analysis of these patients. It showed a 3-year overall survival rate of 100 % in the 69 PMBCL patients evaluated thus far in the UNFOLDER study, of whom half had received either R-CHOP-21 or R-CHOP-14, and half radiotherapy to bulky disease or not. This demonstrates that excellent results can be luckily achieved if the number of patients included in a study is only small enough. Because of the excellent outcome of PMBCL patients with any one of four different strategies in UNFOLDER study, they keep on being recruited to this randomized trial and we see no reason for an intensified chemotherapy regimen such as DA-EPOCH-R for these patients.
4.4 Young High and High-Intermediate Risk Patients (aaIPI ≥ 2)
The first formal proof that rituximab improves also the outcome of this DLBCL subgroup came from the Mega-CHOEP study of the DSHNHL [34]. The Mega-CHOEP study randomized young poor-prognosis (aaIPI = 2–3) patients into 8 cycles of dose-DENSE-R-CHOP-14 (CHOP plus 100 mg/m2 etoposide d1–3) or one cycle of dose-escalated “midi-R-CHOEP” followed by 3 cycles of high-dose (“mega”) R-CHOEP, each necessitating autologous stem cell support. Originally, the Mega-CHOEP study had a second randomization with and without rituximab, but this randomization was stopped when the MInT study demonstrated the efficacy of rituximab in young DLBCL patients. By that time, 31 patients had been randomized not to receive rituximab. Despite of this small number of patients, the difference in 3-year EFS of nearly 30 % (37 % vs. 66 %) was highly significant (p < 0.001) in favor of patients who had received rituximab and the two arms without rituximab were closed (Fig. 3). In Mega-CHOEP patients receiving rituximab, there was no significant difference with respect to 3-year EFS (69.5 % vs. 61.4 %; p = 0.14), PFS (73.7 % vs. 69.8 %; p = 0.48), or OS (84.6 % vs. 77.0 %; p = 0.08) between 130 patients randomized to R-CHOEP-14 and 132 patients randomized to the triple-transplant R-Mega-CHOEP program. Patients with age-adjusted IPI = 2 had a significantly better event-free survival (75.5 % vs. 63.5 %; p = 0.0509) and overall survival (91.0 % vs. 77.1 %; p = 0.01) if treated with R-CHOEP-14, demonstrating that aaIPI = 2 patients have a high cure rate and do not really belong to a “poor-prognosis” subgroup anymore. In contrast, in patients with aaIPI = 3, where no differences were observed between R-CHOEP-14 and the triple-transplant Mega-CHOEP with a 3-year OS of around 75 %, there is still room for improvement.
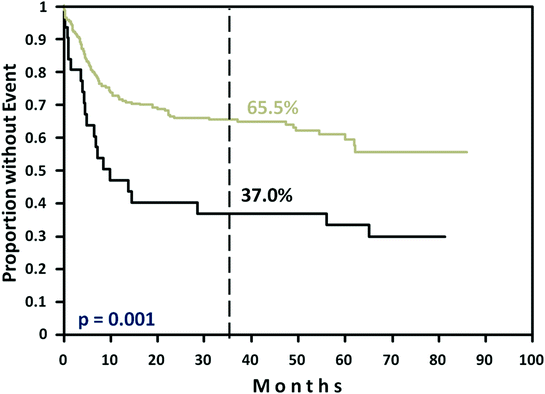

Fig. 3
Event-free survival of young poor-prognosis patients (aaIPI = 2, 3) in the Mega-CHOEP trial [34]. Black curve CHOEP-14/Mega-CHOEP (n = 31); gray curve R-CHOEP-14/R-Mega-CHOEP (n = 262)
Two other studies, so far published only in abstract form, also addressed the role of high-dose chemotherapy and stem cell transplantation in the rituximab era. The French study [35] did not find any advantage of an intensified strategy including high-dose BEAM and stem cell support compared to R-CHOP-14, while R-CHOP-14 followed by a consolidation with 2 cycles of MAD (mitoxantrone, high-dose cytarabine, and dexamethasone) and myeloablative therapy with BEAM was superior to R-CHOP-14 in an Italian study [36] with respect to PFS, but not to OS. This led the authors conclude that high-dose chemotherapy and stem cell transplantation should not be part of a first-line therapy for young poor-prognosis patients in the rituximab era.
The SWOG-9704 study [37] also addressed the role of ASCT in the first-line treatment of young poor-prognosis patients. Patients responding to 5 cycles of (R)-CHOP-21 were randomized to receive either 3 additional CHOP-21 cycles or 1 CHOP-21 followed by ASCT after induction with high-dose BEAM or total body irradiation. One-third of the patients were treated before 2005 and did not receive rituximab. Of 370 induction-eligible patients, only 125 were randomly assigned to the transplantation group and 128 to the control group. 2-year PFS rates were 69 and 55 % (p = 0.005), and 2-year OS rates were 74 and 71 %, respectively (p = 0.30). The 2-year PFS and OS results in both arms of the SWOG study are considerably worse than the 3-year results of any of the two arms of the Mega-CHOEP [34] or the Italian study [36], despite the fact that the survival curves of only those 253/370 patients from the SWOG trial who made it into the randomization are reported, and no results for the overall population recruited to the trial are shown. This means that the respective PFS and OS rates of all 370 patients (including those progressing during the first CHOP-21 or not achieving a response) in the SWOG trial are even considerably worse. The only merit of the SWOG study is that it is the first to evaluate R-CHOP-21 for young poor-prognosis patients in a prospective fashion, and the only lesson that can be learnt from this trial is that R-CHOP-21 is unacceptably ineffective for these patients. Having selected an unacceptably inefficacious comparator arm, all conclusions made by the authors in their publication with respect to the role of ASCT in the rituximab era must be declined.
In summary, while there is no generally accepted standard for young poor-prognosis patients, the results obtained with 8 × R-CHOEP-14 are the best reported to date for this population. This is also supported by a comparison of R-CHOP-14 in the Italian [36] and R-CHOEP-14 in the DSHNHL trial [34] presented at ASH 2013 [38]: Patients treated with R-CHOP-14 had a 3-year PFS of 63 % compared to 74 % of those treated with R-CHOEP-14. In a Cox model, for PFS including treatment arm, age, gender, aaIPI, extranodal sites, bulky disease, and BM involvement, the adjusted HR was 0.68 in favor of R-CHOEP-14, but due to the limited number of patients, this difference was not significant (p = 0.128). Moreover, a retrospective register study from Scandinavia [39] also found a significantly better outcome of young poor-prognosis patients treated with R-CHOEP-14 compared to R-CHOP-14.
For aaIPI = 3 patients, treatment within prospective trials is recommended. R-CHOEP-14 toxicity leaves room for additional drugs for these patients. Since aaIPI = 3 patients make up only roughly 15 % of all young patients, international cooperative efforts are necessary to evaluate innovative concepts within acceptable time frames.
4.5 Patients Aged 60–80 Years
Eight cycles of combination chemotherapy with R-CHOP-21 is the most widely used standard for these patients. Six cycles of R-CHOP-14 plus 2 additional administrations of rituximab or eight cycles of R-CHOP-14 were not superior to 8 cycles of R-CHOP-21 in two prospective randomized studies [16, 40], of which the British trial included all DLBCL patients up to 80 years, while the French included only patients 61–80 years of age. In particular, the French study did not stick to the supportive measures recommended for R-CHOP-14 in elderly patients with DLBCL [26, 41]. This resulted in an unacceptably high therapy-associated death rate of 9 % in the first 100 patients receiving R-CHOP-14 which went down to 2.5 % in the last 200 patients treated with R-CHOP-14 in this trial, indicating a steep learning curve for R-CHOP-14 among the participants of this trial. It can only be speculated on how the results of this randomized study would have looked like if the first 100 R-CHOP-14 had been treated with state-of-the-art supportive measures. With respect to toxicity, no clinically relevant differences were observed between R-CHOP-14 and R-CHOP-21; this was also the case in the British study [16], which included DLBCL patients of any age and IPI. Based on the confirmed equal efficacy and toxicity of R-CHOP-21 and R-CHOP-14, respectively, the 2012 ESMO guidelines recommend either 8 × R-CHOP-21 or 6 × R-CHOP-14 + 2R for DLBCL patients between 61 and 80 years of age. It should be emphasized here that there are no prospective data on 6 × R-CHOP-21 in elderly patients, and in the absence of the latter, we discourage the use of 6 × R-CHOP-21 for elderly DLBCL patients outside clinical trials. R-CHOP-14 has the advantage of a shorter time under chemotherapy (10 weeks compared to 21 weeks with 8 × R-CHOP-21) and therefore is standard for elderly patients in several countries worldwide. 6 × R-CHOP-14 has also the advantage of giving 25 % less doses of doxorubicin and the other cytotoxic drugs, which should have an impact on the rates of cardiotoxicity and second neoplasms with longer follow-up.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree