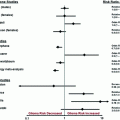
Fig. 1
A schematic overview on the current molecularly based subgrouping. In addition to the standard treatments, radiotherapy (RT), temozolomide (TMZ), procarbazine, lomustine and vincristine (PCV) or radiochemotherapy, there are trials for patients without 1p/19q co-deletion in the tumor tissue (CATNON) and with that molecular alteration (CODEL). isocitrate dehydrogenase (IDH), glioma CpG island hypermethylator phenotype (G-CIMP), radiotherapy (RT), temozolomide (TMZ), procarbazine (PCV), lomustine and vincristine, world health organization (WHO), years (y)
3 Lessons from RTOG 9802, EORTC 26951, and NOA-04/-08 Trials on Patients with Anaplastic Glioma
The most relevant development of the past decade is the delineation of patient groups who have a clinically meaningful response to chemotherapy. Radiographic response to chemotherapy has been reported in up to 70 % of newly diagnosed 1p/19q codeleted oligodendroglioma patients. In the 1990s, two randomized phase III studies for patients with anaplastic oligodendroglioma (AO)/anaplastic mixed oligoastrocytoma (AOA) (RTOG 9402 and EORTC 26951) and one trial in grade II gliomas (RTOG 9802) have been initiated to investigate the added value of PCV chemotherapy to radiotherapy. Another trial, conducted by the NOA (NOA-04) tried to establish monotherapy with temozolomide or PCV instead of radiotherapy in newly diagnosed anaplastic gliomas. In low-grade gliomas, the EORTC 22033 trial aimed to demonstrate superiority of primary temozolomide over radiotherapy.
The initial reports of the EORTC and RTOG studies in anaplastic gliomas, presented in 2006, concluded that there was no significant improvement in overall survival of patients with either codeleted or non-codeleted AO/AOA treated with either intense PCV followed by radiotherapy (RTOG 9402) or with radiotherapy followed by adjuvant PCV (EORTC 26951) (test arms) versus radiotherapy alone (control arm). Already in these final analyses, however, the progression-free survival of codeleted patients treated with the radiotherapy/PCV regimens was significantly longer than with radiotherapy alone [17, 18].
NOA-04 demonstrated that primary chemotherapy with PCV or temozolomide was as effective as primary radiotherapy both in terms of progression-free and overall survival [12]. However, all trials suffered from insufficient follow-up times at the time of the initial reporting and were not able to define the real impact or the appropriate subgroup of patients that benefited the most.
In 2012, more mature long-term survival data for both EORTC 26951 and RTOG 9402 were published which demonstrated an overall survival benefit for patients with 1p/19q codeleted tumors who received a combined radiochemotherapy: EORTC 26951 randomized 368 patients with newly diagnosed anaplastic oligodendroglial tumors to radiotherapy alone or radiotherapy followed by up to six cycles of PCV. Overall survival was 42.3 months with radiotherapy→PCV as opposed to 30 months with RT alone (HR = 0.75, 95 % CI 0.6–0.95). It was not reached versus 112 months in the radiotherapy→PCV versus radiotherapy arms for 1p/19q codeleted tumors (HR = 0.56, 95 % CI 0.31–1.03), but only 25 versus 21 months for non-codeleted tumors (HR = 0.83, 95 % CI 0.62–1.1). Although the addition of PCV significantly prolonged survival (HR = 0.75, 95 % CI 0.60–0.95) in the full trial cohort irrespective of molecular analysis, only the patients with the 1p/19q codeletion derived a clinically relevant OS benefit from the addition of PCV, especially when weighing toxicity of the combined treatment [26]. The data are similar for the North American trial. RTOG 9402 randomized 291 patients with newly diagnosed anaplastic oligodendroglial tumors to RT or RT preceded by up to four cycles of intense PCV. Overall survival was 4.6 years with PCV→RT and 4.7 years with radiotherapy alone (HR = 0.79, 95 % CI 0.6–1.04). Overall survival was 14.7 versus 7.3 years in the PCV→radiotherapy versus radiotherapy arms for 1p/19q codeleted tumors (HR = 0.59, 95 % CI 0.37–0.95), but only 2.6 versus 2.7 years for non-codeleted tumors (HR = 0.85, 95 % CI 0.58–1.23) [27].
Since the assessment of the 1p/19q codeletion does not identify all patients benefitting from radiochemotherapy with PCV in the RTOG 9402 trial, it was tested whether IDH mutations or a germ-line polymorphism, rs55705857, associated with IDH mutant gliomas identified the patients in RTOG 9402 who benefited from combined treatment.
This next retrospective subgroup analysis suggests that patients with 1p/19q codeleted and IDH mutated tumors (14.7 vs 6.8 years; HR, 0.49; 95 % CI, 0.28–0.85; p = 0.01) had the largest numerical benefit from combined radiotherapy and PCV. However, also patients with 1p/19q intact, but IDH mutated tumors showed a relevant benefit, when treated with radiochemotherapy versus radiotherapy alone (5.5 vs 3.3 years; HR, 0.56; 95 % CI, 0.32–0.99; p < .05) [33]. Both, the basis for this benefits, but the question why the EORTC 26951 trial did not suggest a predictive, but a merely prognostic role needs further workup and specifically confirmation by one of the ongoing or future trials in anaplastic gliomas.
Recently, another NOA trial on treatment of anaplastic astrocytoma and glioblastoma in elderly patients confirmed the value of a biomarker for treatment decisions in patients with anaplastic gliomas. The NOA-08 trial enrolled patients older than 65 years and a KPS > 60. Patients were randomized to receive 100 mg/m2 temozolomide given on days 1–7 and days 15–22 (1 week on/1 week off) of a 28 day cycle with the dose being adapted to the actual blood counts, or radiotherapy of 60 Gy administered in 30 fractions of 1.8–2.0 Gy. The primary endpoint was overall survival. The trial had a non-inferiority design. Of 584 patients screened, 412 patients were enrolled, and 373 patients received at least one dose of treatment and were included in the efficacy analyses. Median PFS did not differ between temozolomide [8.6 months (95 % CI, 7.3–10.2)] and radiotherapy arm [9.6 months (95 % CI, 8.2–10.8)] [19].
The value for MGMT status assessment for this group of patients was already suggested by the nonrandomized ANOCEF trial [28] and the retrospective analysis of the German Glioma Network [29]. The randomized NOA-08 [19] and Nordic trials [30] confirmed a predictive role of the MGMT promoter methylation status: In the NOA-08 trial, PFS and overall survival were longer in MGMT promoter-methylated patients who received temozolomide than in those who underwent radiotherapy (8.4 vs 4.6 months), whereas the opposite was true for patients with no methylation of the MGMT promoter (3.3 vs 4.6 months) [19]. In the Nordic trial, overall survival was longer in MGMT-methylated patients who received temozolomide than in those who underwent both radiotherapy regimens (9.7 vs 8.2 months), but similar for patients with no methylation of the MGMT promoter (6.8 vs 7.0 months) [30]. In general, methylation levels outside MGMT promoter methylation are rather low in the tumors of elderly patients and there is a poverty of common positive prognostic factors like IDH mutations [31].
The results from the long-term analyses of the RTOG and EORTC studies led to the suspension of enrolment into the NCCTG-led international intergroup phase III N0577 “CODEL” trial. CODEL was designed to address whether the addition of temozolomide to radiotherapy increased the survival of patients with codeleted tumors. After incorporation of the long-term data into the background of CODEL, the trial “Radiation Therapy With Concomitant and Adjuvant Temozolomide or Radiation Therapy With Adjuvant PCV or Temozolomide Alone in Treating Patients With Anaplastic Glioma“was amended in 2013 to answer the question whether progression-free survival of the combination of radiotherapy and temozolomide is not relevantly different from the combination of radiotherapy and PCV, but potentially harboring less long-term unwanted effects (NCT00887146). Despite its relevance given the general refusal to readopt the PCV regimen together with a lack of data to show that radiochemotherapy with temozolomide is just the same, the trial has not yet generated a momentum and accrual is slow.
At the same time, others are considering trials, in which radiochemotherapy with PCV as a standard is compared to chemotherapy (PCV or temozolomide) alone. Although the temozolomide alone arm of CODEL is designed to address this issue, specifically the timing and extent of neurocognitive health-related quality of life decline in these patients, it is not powered to reach conclusive results. The main focus of a current initiative of the NOA aiming at an international trial with the EORTC is to show superiority of temozolomide alone over partial brain radiotherapy followed by PCV in overall survival without functional deterioration.
Although it is intuitive to think that treating with temozolomide alone while postponing radiotherapy might delay cognitive decline, inferiority of temozolomide alone to the combined initial treatment, i.e., earlier tumor progression in the temozolomide alone arm, might produce the opposite results. Since neurocognitive decline has been shown to correlate with survival of glioma patients and often precedes radiographic progression [32], it is possible that patients treated with temozolomide alone might develop neurocognitive decline earlier than patients treated with radiochemotherapy. Hence, qualification by functional parameters of traditional efficacy endpoints, progression-free and overall survival, is one of the major developments of the upcoming trials. The other challenge is the identification and implementation of biomarkers not only as stratification factors or eligibility criteria in clinical trials, but also into daily clinical practice to determine which patients with a newly diagnosed or recurrent glioma should (or should not) be treated with chemotherapy.
The situation for patients with non-codeleted tumors is more complicated and less favorable. With respect to 1p/19q status, for non-codeleted patients combined radiochemotherapy is not the standard of care. The ongoing “Phase III Trial on Concurrent and Adjuvant Temozolomide Chemotherapy in Non-1p/19q Deleted Anaplastic Glioma: The CATNON Intergroup Trial.“will show whether combined radiochemotherapy with temozolomide (concomitant and/or as an adjuvant maintenance treatment) is superior to radiotherapy alone (NCT00626990).
4 Algorithm for Treatment Decisions in Daily Clinical Practice
The basis for the need for testing 1p/19q, MGMT, and IDH status has already been discussed. In principle, standard of care for patients with an anaplastic oligodendroglial tumor with 1p/19q codeletion is (neo-) adjuvant treatment in addition to radiotherapy. Whether the chemotherapy regimen needs to be PCV or if temozolomide is a suitable alternative might be determined in the amended CODEL trial. Also, data for the exclusion of radiotherapy from the primary treatment may be generated from the long-term analysis of the NOA-04 trial or one of the new CODEL initiatives. Lastly, it is important to understand, whether the data from grade III oligodendroglial tumors can be applied to grade II and/or pure astrocytic tumors.
Recent epigenome-wide analysis, which also allow the determination of copy number aberrations allows classification of anaplastic gliomas into two main categories of G-CIMP+ and G-CIMP-negative tumors. A further subgrouping in the G-CIMP+ was based on 1p/19q status.
Tumor classification based on CIMP and 1p/19q status was significantly associated with survival allowing a better prediction of outcome than the current histopathological classification alone [24].
The data of Cairncross and colleagues on the long-term outcome of RTOG 9402 also justify the treatment of every patient with an IDH mutated oligodendroglial tumor with radiochemotherapy rather than radiotherapy alone [33]. IDH status is a better discriminator of outcome than histological grade in a pooled analysis of grade III and IV malignant gliomas, excluding oligodendroglial tumors [34]. Data from the NOA-04 trial and the German Glioma Network (GGN) demonstrated an interaction between MGMT and IDH status, and with that the basis for the need to test MGMT in patients with 1p/19q intact IDH wildtype tumors. The NOA/GGN analysis demonstrated that in patients with IDH mutations, there is similar benefit from initial RT or alkylating chemotherapy. In patients with IDH wild-type tumors on the other hand, a methylated MGMT promoter status is associated with superior outcome in patients treated with alkylating chemotherapy (with or without additional RT) compared to RT alone [25]. Hence, MGMT is predictive for response to alkylating chemotherapy only in the setting of an IDH1 wild-type glioma. MGMT may be bound and stabilized by factors like N-myc downstream regulated gene (NDRG)1, a central and druggable molecular hub downstream of epidermal growth factor receptor, and mammalian target of rapamycin. It integrates diverse therapy-induced microenvironmental factors to promote resistance toward alkylating chemotherapy. Besides hypoxia and radiotherapy, this is also the use of corticosteroids [35].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree