BMI (kg/m2)
Classification
Waist circumference ≥88 cm
Waist to hip ratio (WHR) ≥0.85
Recommended weight gain in pregnancy
Below 18.5
Underweight
–
–
28–40 pounds
(from 13 to 18 kg)
18.5–24.9
Normalweight
–
–
25–35 pounds
(from 11 to 16 kg)
25.0–29.9
Overweight
High riska
Risk substantially increaseda
15–25 pounds
(from 7 to 11 kg)
30.0 and above
Obese
Very high riska
Risk substantially increaseda
11–20 pounds
(from 6 to 9 kg)
There is still an open debate regarding the concept of obesity as pathology or a simple consequence of overfeeding and physical inactivity. Anyhow, the excess of fat mass in the body should be always considered pathologic when in concomitance with a disease state. Even in the absence of a disease state, fat mass excess is associated with an increased risk of cardiovascular disease [7–9].
In humans we should talk about “obesities” instead of “obesity,” since the same excess of fat mass can be generated by different mechanisms: primary obesity is due to excessive food intake coupled with decreased energy expenditure due to physical inactivity, whereas secondary obesity refers to obesity secondary to endocrine, metabolic, or even genetic disorders. Genetic forms of obesity are rare, explaining at most 3–4 % of human obesities [10–12].
Obesity alters lipid metabolism and it is characterized by a rise in total triglycerides, reduced HDL cholesterol, and an increase of VLDL, although total cholesterol and LDL cholesterol are significantly modified. In obesity also glucose metabolism is altered and characterized by hyperinsulinemia (reaching levels approximately twofold higher than those found in nonobese women) [8]. Besides metabolic complications, it is now widely recognized that obesity is associated with a state of “chronic low-grade inflammation” [13–15]. Serum levels of many pro-inflammatory and anti-inflammatory mediators are chronically altered in obesity, negatively affecting organs such as the liver, skeletal muscle, pancreas, gut, and heart [13–15]. This low-grade inflammation is then viewed as the common pathophysiological mechanism underlying the appearance of chronic and obesity-related complications such as hypertension, type 2 diabetes, metabolic syndrome, cardiovascular disease, and several type of cancers [8, 15–18]. Obesity-linked inflammation is characterized by a modest, but chronic, increase in circulating levels of mediators such as tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6), interleukin 1 beta (IL-1), monocyte chemoattractant protein 1 (MCP-1), and serum amyloid A protein (SAA) together with the increase of leptin and decrease in circulating adiponectin, a cytokine with well-known anti-inflammatory properties [8, 13, 19].
5.2.1 Leptin and Adiponectin
Leptin and adiponectin are also called “adipokines” since these two cytokines are almost exclusively secreted by white adipose cells [19–21]. Leptin and adiponectin have a crucial role in inflammation modulation. Leptin exerts proliferative and antiapoptotic activities in a variety of cell types, including T lymphocytes, leukemia cells, and hematopoietic progenitors, and its levels are acutely increased by inflammatory stimuli, such as in endotoxemia, and by pro-inflammatory cytokines such as TNFα and IL-1 [22]. Adiponectin has anti-inflammatory, antiatherogenic, antioxidant, and antiapoptotic effects, inhibiting TNF-induced endothelial adhesion and macrophage transformation to foam cells and suppressing TNF expression by macrophages, as well as by adipocytes [19, 20]. Of note, even a modest weight loss (5–10 % of weight) is able to positively modulate these inflammatory circulating mediators [23].
5.3 The Molecular Role of White Adipose Tissue in Obesity
During the last decade, white adipose tissue has been recognized as more than simply a storage depot for triglycerides. White adipose tissue is an active endocrine organ secreting adipokines, inflammatory mediators, and many bioactive molecules [19]. Obesity dramatically alters the morphology, as well as the molecular physiology, of white adipose cells [24]. In obesity, adipose tissue is characterized by hypertrophy and hyperplasia of unilocular adipocytes, with a dramatic change in the relative abundance of stromal vascular fraction cells, composed by adipocyte precursors (i.e., preadipocytes), hematopoietic progenitor cells, endothelial cells, and immune cells, 10 % of which are CD14+/CD31+ macrophages [25]. The accumulation of adipose tissue macrophages (ATMs) in visceral adipose tissue of obese individuals has been well described [13, 14]. The number of macrophages in white adipose tissue (WAT) is directly correlated with adiposity and adipocyte mean size, with a higher abundance in visceral than in subcutaneous compartment [14]. The accumulation of T lymphocytes in adipose tissues (ATLs) was also demonstrated, and in mice submitted to high-fat diet, this phenomenon precedes the accumulation of ATMs. Moreover, the increased expression of T-lymphocyte markers was concomitant with the initiation of insulin resistance characterized by a reduction in systemic glucose tolerance and insulin sensitivity [25]. Early ATL infiltration in adipose tissue might be considered as a “primary” event that orchestrates the adipose tissue inflammation. In the adipose tissue of obese subjects, all lymphocyte types were detected: NK (natural killer) and NKT (natural killer T) cells that belong to the innate immune system, B lymphocytes and CD4+/CD8+ lymphocytes that belong to the adaptive immune system, and T lymphocytes responsible for the innate and adaptive immunity [25].
5.3.1 Extracellular Matrix in the Adipose Tissue
In addition to the inflammatory components in the adipose tissue, it should be noted that also the extracellular matrix components are modified in obesity [26]. The extracellular matrix is extremely important for the structure and functions of almost any cell type. Furthermore, it is involved in numerous processes such as cell adhesion, proliferation, differentiation, migration, apoptosis, and gene expression. During obesity progression, the connective fiber content of the adipose tissue increases dramatically, due to an upregulation of several types of collagens. As the collagen content increases, the overall rigidity of adipose tissue also increases, likely contributing to an increase in its mechanical strength. In adipose tissue, “fibrosis” appears to be initiated in response to adipocyte hypertrophy, which occurs as the initial step toward fat expansion through enlargement of the lipid droplet size in adipocytes [26]. The cellular factors associated with adipocyte expansion and collagen activation are currently unknown and a matter of intense investigations, but tissue hypoxia could be certainly involved. Fibrosis of the adipose tissue represents then another pro-inflammatory factor in dysregulation of adipocyte biology. The cellular components of adipose tissue in obesity are summarized in Fig. 5.1.
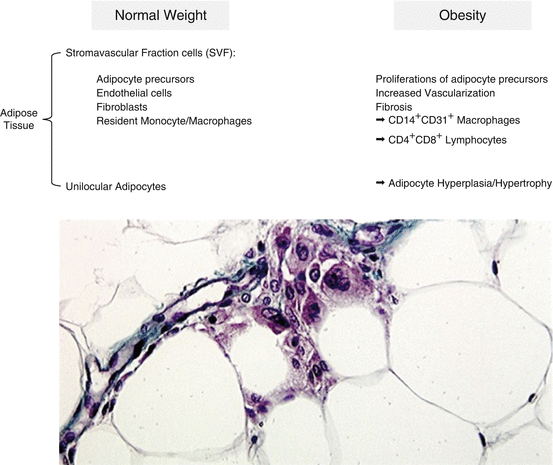

Fig. 5.1
List of cell types in adipose tissue of normal weight vs. obese subjects (upper panel). The lower panel shows the histology of human visceral adipose tissue from an obese donor. Fully mature hypertrophic adipocytes are clearly visible together with inflammatory infiltrations (macrophages and lymphocytes). Green fibers represent adipose tissue fibrosis (60×, Masson’s Trichrom staining; R. Cancello, personal unpublished image)
5.4 Obesity and Inflammation in Pregnancy
As a consequence of the rise in worldwide global obesity, the prevalence of obesity in reproductive-age women is currently 30 % and in the early pregnancy it is about 20 % [27–32]. Pregnancy is a physiological state characterized by changes in weight, maternal fat deposition, and fluid redistribution. An unambiguous assessment of the complications induced by obesity in pregnant women is made difficult by the frequent inability to distinguish the effects of obesity from those due to its complications [33, 34]. It is therefore interesting to focus our attention to the influence that excess of weight has on the mother. The literature shows that pregnancy in obese women is complicated by different medical conditions [33–41], such as:
Higher incidence of adverse pregnancy outcome for the mother and newborn when compared to women with a BMI in a normal range
Type 2 diabetes at the time of conception
Hypertensive disease in pregnancy and preeclamptic toxemia
Mechanical complication (i.e., pelvic pain and lower back pain)
Reduced mobility during pregnancy (increased venous thromboembolism risk)
Overall higher rate of labor induction with higher doses of oxytocin and prostaglandins
Higher cesarean section rates
Macrosomic babies with fat mass excess at birth
Increased risk of postpartum depression
Pregnancy could be considered as a natural “pro-inflammatory” state, as demonstrated by the activation of maternal leukocytes and by mild elevations in circulating levels of both pro- and anti-inflammatory cytokines [39]. It has been suggested that at the local uterine/placental level, implantation (i.e., early pregnancy phase) and delivery (i.e., late pregnancy) may be considered as pro-inflammatory states while mid-pregnancy as an anti-inflammatory state [39]. However, few studies have examined longitudinal changes in circulating cytokines, during pregnancy progression and during postpartum transition.
Numerous studies have shown that maternal obesity is associated with an increased risk of gestational hypertension, but few studies have made a distinction between gestational hypertension and preeclampsia (PE) [35, 36, 41]. A maternal exaggerated inflammatory response to pregnancy, exacerbated by excessive body fatness, is associated with endothelial dysfunction, hypertension, proteinuria, and varying degrees of ischemic end-organ damage that characterizes PE [29–33]. The risk of developing gestational hypertension is directly correlated with maternal BMI [29–33]. However, the results are not entirely in agreement for high maternal BMI and increased risk for development of PE. Several studies show a strong association of maternal obesity with the PE (indicating a doubled risk for every increase of BMI of 5–7 kg/m2), while others denote only a slight tendency in this direction [29–33]. The pathogenesis of vascular damage that underlies PE may also be explained as a manifestation of a state of insulin resistance.
A condition of hyperinsulinemia, typical of obesity, is able to alter the intracellular cationic pumps regulating vascular tone and blood pressure, to stimulate the sympathetic nervous system and to induce hypertrophy of smooth muscle cells [42–44]. An increased level of vasoactive peptides that is associated with hyperinsulinemia may then contribute to endothelial damage that is characteristic of PE. In confirming a strong correlation between obesity and the onset of PE, several authors have emphasized a common condition: the activation of the inflammatory system [42–44]. The finding of high serum concentrations of IL-6 and CRP in obese pregnant women is a key point in the condition of chronic inflammation typical of obesity, which correlates negatively with endothelial function and positively with the levels of fasting insulin, thus demonstrating a possible implication in the pathogenesis of gestational diabetes mellitus (GDM) [44, 45].
From these observations it emerges as the metabolic and inflammatory features of obese women may predispose to vascular damage then helping the mechanism through which the maternal adiposity is associated with the emergence of PE. Epidemiological studies demonstrated that women with pregnancies complicated by PE are more likely to develop coronary heart diseases later in life [29, 30]. However, TNF and IL-6 expression, well-known acute-phase reactant, are increased in obese pregnant women independently of PE, then suggesting the involvement of other sources to the circulation than the placenta itself. A role for maternal adipose tissue, particularly visceral fat, could then be suggested [8].
Abdominal obesity is frequently associated with a condition of hyperinsulinemia. High concentrations of insulin reduce the number of insulin receptors and, as a consequence, the effect of insulin itself. The maintenance of a euglycemia condition therefore requires a progressive increase in insulin secretion. Pregnancy, which is a condition of stress on carbohydrate metabolism, may affect this delicate balance. Multiple studies document an increased risk of GDM among obese pregnant women, in particular between those with morbid obesity (BMI > 35 kg/m2) [42–46]. An incidence of 24.5 % for GDM in pregnant women with BMI greater than 40 kg/m2 compared to 2.2 % in women with BMI between 20 and 24.9 kg/m2 was reported [42–46]. Women who develop GDM are also more likely to develop diabetes in their lifetime [29]. Such considerations make it necessary to identify patients potentially at risk of developing GDM, in order to implement preventive measures. It is demonstrated that even a modest weight loss between two subsequent pregnancies modifies the risk of GDM development [42–46]. Obesity finally exposes pregnant women to a worsening of preexisting diabetes with an increased risk of fetal macrosomia, nervous system defects, cardiovascular diseases, and increased mortality risk, as well as an increased likelihood of developing obesity in childhood [29, 32–34].
5.5 Adipose Tissue in Pregnancy
The functional/morphologic changes of adipose tissue in pregnancy have been poorly studied in humans, but it is possible that the obesity-linked metabolic and inflammatory adipose tissue alterations represent an additional factor contributing to a pro-inflammatory state in pregnancy [35]. High subcutaneous abdominal adipose tissue thickness during pregnancy has been associated with elevated inflammatory marker levels [46]. It was described that abdominal thickness ≥15 mm (by ultrasounds techniques) is a strong predictor of high CRP and HbA1c circulating levels in pregnant women [47]. In addition, higher values of fat thickness during 24–28 weeks of gestation are associated with pregnancy-related complications that could be observed during later periods of gestation [47, 48]. Measurement of abdominal fat might then be helpful to identify groups at risk [47, 48].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree