Nutrition Support
William A. Mourad
Caroline M. Apovian
Christopher D. Still
Malignant disease is frequently accompanied by profound weight loss and malnutrition. In fact, cancer patients have the highest prevalence of malnutrition of any hospitalized group of patients (1). In its most severe form, weight loss due to malignancy is termed the anorexia-cachexia syndrome and is characterized by anorexia, skeletal muscle atrophy, tissue wasting, and organ dysfunction (2). Malnutrition associated with malignancy is an indicator of a poor prognosis. It is associated with a higher mortality rate (3) and higher perioperative morbidity in severely malnourished patients (4, 5, 6). However, it is unclear if the routine use of nutritional support in cancer patients can mitigate those consequences. A great deal of research has attempted to answer this question and guidelines for use of nutrition support have been set up by the American Society for Parenteral and Enteral Nutrition, the American Gastroenterological Association, and the American College of Physicians (7, 8, 9).
Choosing the appropriate route of nutrition support for a malnourished patient is an important decision predicated on the patient’s clinical situation. Although enteral feeding is the preferred route for patients with a functional gastrointestinal (GI) tract, many patients have relative or absolute contraindications to and are unable to tolerate enteral feedings (5, 7, 8, 9). Patients with severe diarrhea, abdominal distention, high nasogastric tube output, or unobtainable safe access to the GI tract are poor candidates for enteral nutrition. The complications of enteral nutrition include pulmonary aspiration, diarrhea, and intestinal ischemia or infection. The risks of parenteral nutrition include infection, electrolyte imbalances, and a failure of large controlled studies showing an improvement except in selected patient populations (the severely malnourished surgical patient and bone marrow transplant patients) (4, 5, 6, 7, 8, 9, 10).
This chapter will address the following questions: what is the effect of nutrition support on the outcomes of patients with cancer undergoing various treatment modalities? What is the role of nutrition support in the treatment of cytokine-mediated cancer anorexia and altered host metabolism? Are there specific nutritional supplements that take on a pharmacologic role by modulating tumor cell growth? Finally, is preservation of quality of life, by reduction of fatigue and other effects of malnutrition, an adequate outcome of adjuvant nutritional therapy? Because of the ongoing controversies associated with these issues and ethical issues regarding nonvolitional feeding guidelines, nutrition support of the patient with cancer should be emphasized in the clinical setting. Nonetheless, nutrition support is an integral component of comprehensive cancer care in patients who are carefully selected through the use of nutritional screening tools and for whom appropriate goals of treatment have been established.
Malnutrition in the Cancer Patient
Malnutrition in the patient with cancer results from several mechanical and metabolic processes. The cachexia of malignancy is a complex metabolic disorder characterized by involuntary weight loss which, if not treated, often leads to death (3). Certain tumors predispose individuals to cancer cachexia. Table 67.1 depicts the frequency of weight loss among approximately 3000 patients with cancer studied by the Eastern Cooperative Oncology Group. Breast cancer and sarcomas rarely resulted in significant host weight loss compared with cancers involving digestive organs such as stomach and pancreas. Patients with lung cancer or prostate cancer also demonstrated significant weight loss and malnutrition. The Eastern Cooperative Oncology Group survey demonstrated a lower morbidity and longer survival in patients without weight loss, except in cases of pancreatic or gastric cancer. The role, however, of nutritional support preventing weight loss remains unclear.
Causes of Malnutrition
Table 67.2 lists the potential etiologic factors in the development of malnutrition and cancer anorexia. The development of malnutrition in the individual patient is usually the result of a combination of these mechanisms (11).
Anorexia of Malignancy
Several factors, including changes in the central nervous system, changes in taste perception due to tumor, depression, and its associated reduced physical activity, have been implicated as causes of cancer anorexia. The regulation of appetite is complex and is mediated by blood nutrient levels, host nutrient resources, liver function, GI capacity or toxicity, medications, and environmental cues (7, 8, 11, 12). When weight loss is encountered despite adequate caloric intake, it is termed the anorexia-cachexia syndrome. Recently, considerable attention has been directed to the role of various cytokines and tumor byproducts as mediators of this syndrome which are discussed in detail later in this chapter under mediators of cancer cachexia.
Table 67.1 Frequency of Weight Loss in Cancer Patients | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nutritional Effects of Cancer Treatments
Radiation
The site, magnitude, and duration of radiation therapy all influence the severity of nutritional compromise. This injury may be associated with both acute and long-term effects of radiation therapy (13, 14).
After radiotherapy of the oral cavity and pharynx, patients can experience either heightened or suppressed taste sensation. Loss of taste is severe and rapid after oropharyngeal irradiation, as measured by quantitative tests of sensitivity to sour, sweet, and bitter substances. Fortunately, patients often regain preradiation taste sensitivity 60 to 120 days after therapy is completed.
Radiation to the head and neck may inhibit adequate salivation, leading to changes in eating habits. Also, patients may experience increased sensitivity of their teeth to extreme temperature and sweetness. In a study of weight loss during a 6- to 8-week course of external beam radiation therapy to the head and neck regions, 93% of 114 patients lost an average of 3.7 kg. In addition, approximately 9% of individuals lost more than one tenth of their body weight during their 6- to 8-week course of radiation (14).
Table 67.2 Etiology of the Anorexia-Cachexia Syndrome | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Radiation to the gastric area and small and large intestine is associated with various complications that may influence nutritional status. Low doses of gastric irradiation reduce gastric acidity whereas higher doses may induce ulcer formation. Nausea, vomiting, and diarrhea are common in individuals undergoing radiation to the small and large bowel. In addition, chronic diarrhea or bowel obstruction may develop due to radiation-induced enteritis following high-dose GI irradiation.
Upper abdominal radiation may produce radiation-induced hepatitis, characterized by anorexia, nausea, vomiting, and abdominal distention. This disorder is usually temporary. Radiation to the pancreas may similarly result in acute anorexia, nausea, and vomiting.
Chemotherapy
Chemotherapy can affect host tissue in addition to the targeted neoplasm and thereby cause short-term nutritional defects. Many chemotherapies and immunotherapy products can produce nausea, vomiting, and diarrhea. This can lead to anorexia, electrolyte imbalance, and malnutrition.
Mucosal toxicity, manifested as oral ulcerations, cheilosis, glossitis, and pharyngitis, may be inevitable with the use of some chemotherapeutic agents. This often leads to odynophagia and anorexia. Specific agents, such as actinomycin D, cytarabine, 5-fluorouracil, hydroxyurea, and methotrexate, can produce ulcerations of the entire GI epithelium (15). Therapy itself does not induce malabsorption but, because of its side effects, can aggravate the effect of tumor-related malabsorption syndromes (15). With either a single agent or combination chemotherapy, small intestinal absorptive function remains well preserved in most instances. Nutrition support is usually unnecessary in the initially well-nourished patient if the ill effects of chemotherapy are significantly limited in time and intensity.
Multiple major organ systems are affected by chemotherapeutic agents which results in decreased efficiency of metabolism. The liver is especially vulnerable, and hepatic injury is commonly associated with anorexia. Diffuse hepatocellular damage often results in hypoalbuminemia. Several additional agents affect other organ systems. Hydroxyurea may cause renal impairment. Doxorubicin may cause cardiac toxicity leading to congestive heart failure, water retention, and electrolyte imbalance. Carter (16) provides extensive discussions of the nutritional consequences of chemotherapy and immunotherapy.
Surgery
Nutritional depletion can be attributed to surgical intervention in many cancer patients. Nutrition support can be a beneficial tool, as a patient with good nutritional status develops fewer postoperative complications. Poor nutritional status can affect wound healing immune function and several studies have shown that when patients with severe malnutrition receive nutritional support the complication rate improves (4, 5, 6).
Certain surgical procedures may require nutritional intervention. Surgery of the upper GI tract can compromise a patient’s ability to take oral feedings. Conditions associated with esophagectomy and esophageal reconstruction may include delayed gastric emptying secondary to vagotomy, malabsorption, and the development of a fistula or stenosis. Gastric surgery may result in dumping syndrome, malabsorption, and/or hypoglycemia.
The site and extent of intestinal resection can result in a variety of nutritional complications. Jejunal resection can decrease the efficiency of absorption of carbohydrates, protein, fat, water, and electrolytes. Ileum resection commonly results in vitamin B12 deficiency and bile salt losses. With extensive small bowel resection, malabsorption can result. Abnormalities in sodium and water balance are commonly associated with ileostomy and colostomy formation. In addition, gastric and intestinal bypass surgery to relieve obstruction can result in a blind loop syndrome with specific nutritional deficiencies.
Individuals with pancreatic cancer should be nutritionally assessed because they frequently lose weight before their diagnosis and surgical intervention. After pancreatectomy, malabsorption as well as endocrine and exocrine insufficiency are common problems that may require insulin and pancreatic enzyme replacement. Patients with cancer undergoing uretero-sigmoidostomy may experience hyperchloremic acidosis and hypokalemia in addition to other more common postoperative problems.
If it is anticipated that nutritional intervention will be required postoperatively, a nasoenteric feeding tube can be placed intraoperatively. With patients in whom a longer recuperative course is anticipated, surgical placement of a gastrostomy tube or jejunostomy tube may be more appropriate.
Carbohydrate Metabolism
Abnormal glucose metabolism, a major source of caloric wasting, is a hallmark of cancer cachexia (17). Both, increased glucose production and utilization as well as insulin resistance are characteristic. Glucose intolerance may be attributable to a reduced tissue sensitivity to insulin (17). There may be decreased pancreatic β-cell receptor sensitivity in patients with cancer as well, leading to inadequate insulin release in response to a glucose load. Such abnormalities in peripheral glucose metabolism simulate a type II diabetic state and also share elements similar to the stress state (17).
An increase in gluconeogenesis in the liver of the patient with cancer is a common finding. This can be the result of an increased release of glucogenic fuel substrates, such as lactate and alanine, as well as an increased induction of hepatic gluconeogenetic enzymes (17, 18, 19). The energy drain created by increased hepatic gluconeogenesis leads to host depletion of protein and calories. Elevation level of hepatic glucose production in a tumor-bearing host requires energy; three moles of adenosine triphosphate are necessary to convert each mole of lactate to glucose. Therefore, abnormal glucose kinetics can result in calorie-consuming futile metabolic cycles, with a drain on host energy. Patients with cancer who lose weight may exhibit increases in glucose flux and glucose oxidation rates. This mechanism is central in the development of host depletion in cachexia, although the energy expenditure involved in these processes suggests that they are more likely associative than causative (20, 21).
Protein Metabolism
Cancer anorexia is accompanied by wasting of host protein mass, leading to fatigue, weakness, and altered host cell-mediated immune response. Clinical signs include skeletal muscle and visceral organ atrophy, hypoalbuminemia, and anergy. Host protein wasting results from alterations in total body protein turnover, muscle protein synthesis and catabolism, hepatic protein metabolism, and plasma secretory protein levels. Several studies have shown that cachectic cancer patients have elevated rates of protein turnover and that the normal adaptation of reduced protein metabolism (i.e., protein sparing) in the setting of starvation does not occur (22). With nutritional depletion, patients with cancer anorexia continue to manifest elevated rates of skeletal protein mobilization rather than the normal response of decreased protein turnover and protein sparing. Protein turnover rates in cachectic patients with cancer are significantly higher compared with similarly malnourished control patients without cancer. A loss of less than 100 g of protein nitrogen or 625 g of body protein equal to 2.5 kg of lean body mass can produce mild protein malnutrition, fatigue, weakness, and altered immune response (23). Explanations for this phenomenon of increased nitrogen turnover remain controversial. However, the findings suggest an injury response to cancer with the tumor acting as a “nitrogen sink.”
Lipid Metabolism
The most common impairments in lipid metabolism seen in patients with cancer are hyperlipidemia and depletion of fat stores (24, 25, 26). Abnormal lipoprotein lipase activity alters the ability of fatty acids to be utilized as protein-sparing energy fuels and is a major cause of protein malnutrition. The hyperlipidemia seen in patients with cancer is significant and may play a role in disease outcome. Elevated lipid levels may have an inhibitory effect on monocytes and macrophages. The combination of increased lipolysis, fatty acid recycling through very low density lipoprotein, and the impairment of lipoprotein lipase enzyme activity leading to decreased lipid clearance can be immunosuppressive.
Suppression of lipoprotein lipase occurs in patients with cancer, but the mechanism is different from that which occurs in starvation. In the latter, lipid mobilization occurs despite a decrease in lipoprotein lipase activity because of a 50% decrease in plasma insulin levels. Vlassara et al. (27) have shown that cancer-associated reductions in plasma lipoprotein lipase are accompanied by normal or even increased insulin levels. This represents a maladaptive host response, since insulin promotes lipid storage, not fat oxidation. Impaired glucose and fat oxidation provide a sink for loss of gluconeogenic amino acids, therefore producing an energy deficit and malnutrition.
Biochemical Mediators of the Anorexia-Cachexia Syndrome
The anorexia-cachexia syndrome incorporates a group of symptoms and signs such as inanition, severe anorexia, weakness, skeletal muscle wasting, organ dysfunction, and progressive weight loss. Physiologically it is characterized by dramatic loss of triglycerides from adipose tissue and loss of visceral and skeletal protein mass (28). Potential etiologies of cancer anorexia are listed in Table 67.2. Cachexia was previously thought to be the result of increased energy demanded by the growing tumor mass. Recent research has demonstrated that it is primarily due to immune mediators [e.g., tumor necrosis factor (TNF), interluekin-6, and eicosanoids], and tumor byproducts (e.g., proteolysis—inducing factor, lipolytic hormone) causing abnormalities in carbohydrate, protein, and lipid metabolism (11). This means that anorexia, an almost universal characteristic of cachexia, should be interpreted as the result of metabolic abnormalities rather than the main cause of cachexia, and that the weight loss seen is not simply a result of decreased caloric intake. In addition to risks that worsening nutritional status has on complication rates of surgery, radiation therapy, and chemotherapy, the cachexia and anorexia are also a source of psychological distress for patients and their families (12).
There has been much interest in the possible role of cytokines in cancer anorexia. Prominent cytokines include TNF, interleukin-1α, and β (IL-1), interleukin-6 (IL-6), interferon-α (IFN-α), and differentiation factor, also known as leukemia inhibitory factor (29, 30). The peptides are pyrogens, and their administration, with the exception of IL-6, can produce many of the systemic vascular effects seen in sepsis and shock. For example, IL-1 and TNF-α share the capacity to up-regulate gene transcription of endothelial cell adhesion molecules, increase procoagulant activity, promote the transendothelial migration of leukocytes out of the vascular component into the pulmonary epithelium, and activate the release of toxic superoxide and other enzyme products from neutrophils (31).
Features of cancer cachexia can also be reproduced by cytokine administration. For example, IL-1 (32) and, to a lesser extent, TNF-α (33) and IFN-α (34) are potent anorexia-producing agents. Some cytokine effects are mediated through the hypothalamus (32), and others act directly on the GI tract, causing decreased gastric emptying (33). Other cytokines may promote cachexia by increasing resting energy expenditure. Warren et al. (35). reported that patients with cancer receiving cytokines as antineoplastic therapy had a dose-dependent increase in resting energy expenditure.
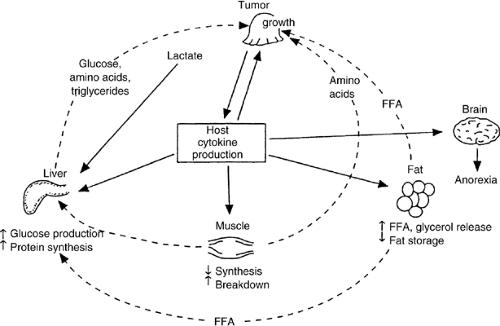
A mechanism proposed by Kern and Norton (2) to explain the anorexia and metabolic derangements of the anorexia-cachexia syndrome is shown in Figure 67.1. Some cancers incite a paracrine-induced systemic host response with production of cytokines such as interleukins or cachectin/TNF. These are secreted by immune cells and may be part of the host defense in an attempt to destroy the tumor. However, cytokines have negative secondary effects on host organs, resulting in anorexia and abnormalities in carbohydrate, protein, and lipid metabolism. Mobilization of nutrients from fat and skeletal muscle during the acute phase of sepsis or in patients with trauma rapidly provides a physiologic source of nutrients to the liver so that it can synthesize acute injury proteins. In a patient with cancer, the low-grade release of cytokines persists because of the metabolic activity of the tumor and eventually causes severe depletion of host cell mass, skeletal muscle wasting, and abnormal lipolysis. The anorexia of chemotherapy, radiotherapy, or surgery only exacerbates this process, adding to the net effect of host depletion. The “at-risk” patient with cancer can be identified by a rapid weight loss of greater than 10% of usual body weight.
There are other prominent mediators aside from cytokines that appear to play a prominent role in skeletal muscle wasting. A 24-kilodalton proteoglycan, called proteolysis-inducing factor (PIF), was identified in the murine MAC16 colonic adenocarcinoma model of anorexia. This factor was subsequently identified in the urine of weight-losing patients with cancer but not in the urine of weight-stable patients with cancer or weight-losing controls with benign disease (29, 36, 37). There is also some evidence that eicosanoids play a role in the metabolic affects PIF and affect protein and fat metabolism (38, 39).
Although the murine MAC16 model demonstrates muscle wasting to PIF and not to certain cytokines, there are other models that show similar metabolic activity with cytokine production but do not demonstrate activity of PIF. One final pathway of these multiple mediators of skeletal muscle
catabolism could be the ATP-ubiquitin-proteasome pathway. In fact, animal studies have shown that both TNF and PIF can increase proteasome activity (38, 40, 41). This suggests that these multiple mediators may share a common downstream affect on catabolism (11).
catabolism could be the ATP-ubiquitin-proteasome pathway. In fact, animal studies have shown that both TNF and PIF can increase proteasome activity (38, 40, 41). This suggests that these multiple mediators may share a common downstream affect on catabolism (11).
Fordy et al. reported that patients with significant weight loss from colorectal liver metastases required a large tumor volume (42). This weight loss was not explained by changes in diet, quality of life, or hormones, but activation of the innate immune systems and incomplete activation of the acquired immune systems were likely to be involved. Agents that attenuate either the acute-phase inflammatory response or T lymphocyte IL-2 receptor up-regulation might reduce weight loss in patients with metastatic disease (42). Some studies have looked at targeting other inflammatory mediators like eicosanoids with NSAIDs or COX2 inhibitors as a means of preventing weight loss (43). The final role anticatabolic medications will play in reversing the weight loss seen in cancer cachexia has yet to be determined.
Preventing malnutrition is easier than reversing it. Reversing malnutrition in a patient with advanced cancer is nearly impossible. Patients with cancer often lose weight and become malnourished at a time when they most need nutrition support (44). Current pharmacologic therapies as well as complementary and alternative methods may be recommended for patients with cancer cachexia (45). In recent years, anabolic medications, such as corticosteroids and progestational drugs, have proven effective in relieving symptoms of cancer cachexia. Corticosteroids have a limited effect (lasting up to 4 weeks) on symptoms such as appetite, food intake, sense of well-being and performance status, but none of the studies proved that patients had weight gain (12). Recent studies involving terminally ill patients have shown that progestational drugs improved the symptoms of fatigue and anorexia even in the absence of weight gain. If abruptly discontinued, both megestrol and medroxyprogesterone could induce thromboembolic phenomena, breakthrough vaginal bleeding, peripheral edema, hyperglycemia, hypertension, Cushing’s syndrome, alopecia, adrenal suppression, and adrenal insufficiency. However, in most clinical trials, the adverse effects rarely led patients to discontinue these drugs. There has also been much interest in appetite stimulants (Cannaboids) and anticatabolic medications (eicosapentaenoic acid, pentoxifylline), but the most consistent data so far has been for megestrol and medroxyprogesterone (12, 46).
Adjunctive Nutrition Support During Antineoplastic Treatment
The goal of nutritional care in the cancer patient should always be considered supportive whether the aim of primary therapy is cure or palliation. Nutritional therapy should be aimed at improving metabolic status, body composition, functional status, and, ultimately, quality of life. Nutrition support can prevent further deterioration in all of these parameters. However, because of the metabolic derangements of cancer cachexia, attempts to reverse severe nutritional depletion are almost universally unsuccessful.
A concern also exists about the possibility of disproportionately stimulating tumor growth while trying to replete the malnourished cancer patient, but recent work has not supported this theory (47, 48). Undoubtedly in a cachectic noncancer patient receiving either total parenteral nutrition (TPN) or enteral feeding, nitrogen balance, wound healing, and outcome are improved. However, controversy continues about the role of nutrition support in patients with cancer. In the setting of chemotherapy, the risk of providing nutrition support inappropriately must be foremost in clinical decision making. TPN is not indicated for patients with advanced metastatic cancer who are not receiving antineoplastic treatment, and the routine use of TPN in patients with cancer who can tolerate enteral nutrition is unjustified. The potential adverse consequences of nutrition support in the setting of cancer make it important to establish the therapeutic benefits before the initiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree