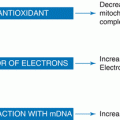
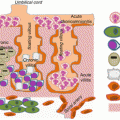
Fig. 11.1
Hypertension in response to placental ischemia: proceeds via immune activation, CD4+ T-cells mediating the release of angiotensin II type-1 receptor autoantibody (AT1-AA), and inflammatory cytokines that contribute to the increased vasoactive peptide ET-1 increased sensitivity to ANGII, oxidative stress, and sFlt-1, all known players in the pathophysiology of preeclampsia (Modified from La Marca et al. [75])
The following paragraphs will highlight the importance of inflammatory cells and products to cause the characteristic rise in blood pressure and decline in renal function that occur during placental pre-eclampsia.
11.3.1 Natural Killer Cells
Natural killer (NK) cells play an important role in the innate immune response providing viral protection and efficiently killing tumor cells by secreting granulosymes and cytotoxins.
NK cells compose a large portion of uterine lymphocytes from which they are distinguished by specific markers such as CD56 bright [64, 65]. These cells secrete angiogenic cytokines and proteins such as angiopoietin 1 and 2 and vascular endothelial growth factor (VEGF) and placenta growth factor (PlGF) [65]. Uterine NK cells prefer close association with the trophoblasts and secrete cytokines that play an important role in trophoblasts differentiation, growth, and spiral arteriole invasion during normal pregnancy and thus contribute to the success of trophoblasts invasion [64, 65].
Uterine NK cells can induce lysis of trophoblast cells lacking specific cellular surface antigens that would normally invade the spiral arteries. Incomplete loss of such cells results in the shallow trophoblast invasion and thus the deficient oxygen and nutrient supply to the developing placenta, which has been described as the genesis of placental pre-eclampsia. Furthermore, during pre-eclampsia inadequate vasculogensis of the placenta leads to hypoxia, thus stimulating production of the VEGF and PlGF antagonist sFlt, thereby stimulating a viscous cycle of events that worsens throughout the pregnancy. These data indicate the importance of the functional profile of the uterine NK cell to either maintain or compromise a potentially healthy pregnancy.
11.3.2 CD4_ T Helper Lymphocytes
The maternal immune tolerance involves crucial interactions between regulatory CD4_T cells and uterine NK cells recognizing and accepting the fetal antigens and facilitating placental growth. Complete failure leads to poor placentation and dysfunctional placental perfusion and chronic immune activation originating from the placenta. Analysis of blood collected from preeclamptic women has demonstrated a decrease in the proportion of circulating regulatory CD4_ T cells [66, 67].
11.3.3 B Lymphocytes
An important function of CD4_ T cells is to facilitate the B lymphocyte memory immune response and specific antibody production toward a single antigen. This process is known as the T-cell-dependent antibody response [68].
Auto-antibodies are produced during pre-eclampsia, suggesting an important role for B lymphocytes in the pathogenesis of this disease. Moreover, Liao et al. demonstrated that the percentage of circulating memory B-lymphocytes were significantly greater in preeclamptic women than in the Non Preeclamptic (NP) cohort [69]. B-2 B lymphocytes are the conventional memory B cells that undergo antigen processing via recognition of MHC class II peptide complex with the activated CD4_T lymphocyte [68]. For B-cell maturation and IgG production, several co-stimulatory signals must occur between the antibody producing B lymphocyte and CD4_ T-helper cell [68]. One of these includes stimulation of the CD20 receptor on the surface of the B cell. This recognition stimulates the B cell to enter the circulation and produce antigen-specific immunoglobulin. Another necessary co-stimulatory molecule for B-cell maturation is the CD40 on the surface of the B cell. This molecule binds with the CD40 ligand on the surface of the T cell [68]. B cells then proceed through proliferation, differentiation, and internal isotype switching, inducing to production of specific antigen-stimulated antibodies, which leads to the formation of short-lived plasma cells that secrete antibody and memory B cells residing in the germinal lymph node centers, which will be available for future interactions with specific T cells. B lymphocytes can be characterized as either B1 or B2 cells, each having distinct markers and roles in facilitating immune reactions. B1 lymphocytes can be divided into B1a or B1b cells [68, 69]. These cells express IgM in greater quantities than IgG and are the primary source of natural antibodies produced in the absence of antigenic stimulation. These antibodies are polyreactive and cross-react with multiple antigens such as autoantigens, other immunoglobulins, and bacterial polysaccharides. Recently, Jensen et al. uncovered an important role for B1 lymphocytes in the progression of pre-eclampsia [70]. Preeclamptic placentas stained positive for markers of B1 B lymphocytes (CD19_CD5_). Furthermore, these authors demonstrated that B1 lymphocytes were stimulated to produce AT1-AA when co-cultured with sera from preeclamptic women but not from normal pregnant women. This study further illustrates the importance of B cells in the preeclamptic placenta and their stimulation by a soluble factor to produce AT1-AA and contribute to the progression of this disease. Furthermore, high levels of B1 cells is yet another important characteristic that preeclamptic women share with patients presenting with autoimmune diseases.
11.3.4 Activating Autoantibodies to the Angiotensin II Type I Receptor (AT1-AA)
Many studies in preeclamptic women have demonstrated increased circulating concentrations of an agonistic autoantibody to the angiotensin type 1 receptor (AT1-AA). AT1-AAs are implicated as a central mediator of several pathophysiological mechanisms in pre-eclampsia. During pre-eclampsia, AT1-AA induce NADPH oxidase and the MAPK/ERK pathway leading to NF-_B and tissue factor activation. AT1-AA stimulate sFlt-1 expression from trophoblast cells and IL-6 production from mesangial cells; they cause increased intracellular Ca2_ signaling in platelets in women who went on to develop pre-eclampsia (Fig. 11.2) [65, 68, 71].
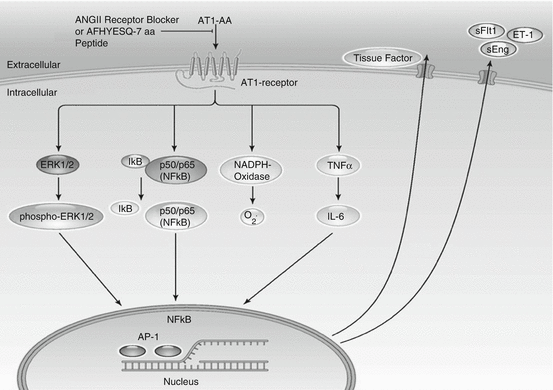

Fig. 11.2
Signal cascades of the angiotensin II type 1 receptor autoantibodies. AT1-AA induce signaling by the angiotensin II type 1 receptor (AT1-receptor), inhibited by AT1-receptor blocker (ARB) or the seven-amino acid peptide (AFHYESQ) mimicking the epitope of the AT1-AA in the second extracellular domain of the AT1-receptor. Intracellular cascades and promoter activations in the nucleus lead to an upregulation of endothelin-1 (ET-1), tissue factor, soluble fms-like tyrosine kinase-1 (sFlt1), soluble endoglin (sEng), and oxidative stress (Modified from La Marca et al. [75])
11.3.5 Inflammatory Cytokines
Several studies have shown an important role for inflammatory cytokines such as IL-6, IL-17 and TNF-α in the etiology of pre-eclampsia.
Studies in animal models have been important to show that moderate, long-term increases in cytokines during pregnancy raise blood pressure and compromise renal function. Mechanisms of hypertension during pregnancy in response to elevated cytokines appear to involve activation of ET-1, increased oxidative stress, and activation of AT1 receptors by AT1-AA. In addition, many laboratories have shown that TNF-α directly stimulates endothelial cells in culture to secrete ET-1 and sICAM, thus attracting leukocytes to adhere to vascular tissues determining edema, which could lead to temporary increases in blood pressure [67]. TNF-α mRNA is increased in preeclamptic placentas and thus could directly increase ET-1 in the placental unit.
IL-6 is important in both anti-inflammatory and pro-inflammatory processes and is a pivotal cytokine to influence activation of B cells as well as effector or regulatory T cells [68]. IL-6 is elevated in preeclamptic women, and AT1-AA-induced hypertensive pregnant mice.
IL-17 is a cytokine that has mostly been associated with autoimmune diseases but has recently gained attention in the aetiology of pre-eclampsia [67, 68]. Recent studies have shown that circulating IL-17 secreting TH17 cells are increased in preeclamptic patients compared with non pregnant patients [67]. Additionally, an important role for TH17 cells and IL-17 in the clearing of bacterial infections has been shown [68]. One important function of IL-17 producing TH17 cells is to recruit neutrophils and other phagocytic cells to a site of infection. IL-17 stimulates neutrophil activation, production of antimicrobial substances such as defensins and ROS, and phagocytosis of microbes or necrotic tissues [68]. Macrophages and neutrophils convert molecular oxygen into ROS by the phagocyte NADPH oxidase system. Activated neutrophils cause injury to normal host tissues, such as the placental unit, by the release of lysosomal enzymes, ROS, or nitric oxide. Preeclamptic women display oxidative stress, increased NADPH oxidase subunits within the placental unit, and elevated blood, urinary, and placental 8-isoprostanes, an indicator of whole body oxidative stress.
An additional cytokine gaining attention in the area of preeclamptic research is CD40/CD40 ligand. The CD40 antigen binds to the CD40 ligand on T cells and is important to stimulate B-lymphocyte proliferation, as previously mentioned [68]. A recent study compared the effect of maternal serum from preeclamptic patients and NP patients to induce apoptosis in cultured endothelial cells [72]. This study showed that endothelial dysfunction may be induced by this CD40/CD40 ligand. These authors found that altered morphology, decreased cell growth, and increased apoptosis were greater with CD40/CD40 ligand increased expression following exposure to preeclamptic sera versus sera from healthy normal pregnant women. However, in vivo studies revealing an important role for CD40/CD40 ligand during pregnancy are lacking. Furthermore, inhibition of this interaction would inhibit T cell-B cell communication and could clarify the role of either memory B cells vs. non memory B cells in the production of AT1-AA, as mentioned previously. Non memory B cells do not require this interaction with T cells for antibody secretion. Therefore, this could be a defining study revealing a role for the memory immune response as well as the route of production of AT1-AA during pre-eclampsia (Fig. 11.3).
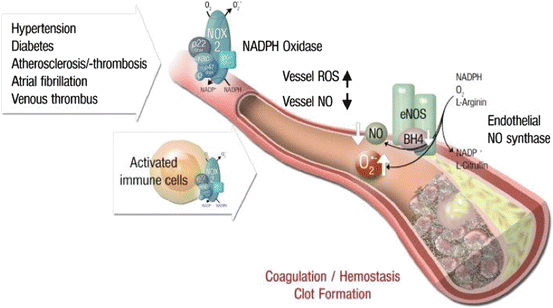

Fig. 11.3
Inflammatory cells, vascular dysfunction and atherothrombosis. The scheme illustrates the activation of immune cells and recruitment to vascular tissues leading to activation of secondary RONS sources such as NADPH oxidase and uncoupled eNOS, all of which contributes to vascular dysfunction. These processes lead to late-stage cardiovascular complication such as atherosclerosis with plaque formation and thrombosis. Vice versa, vascular RONS can activate immune cells and Trigger their Infiltration into the vascular wall (Modified from Karbach et al. Curr Pharm Des 2014)
References
1.
Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Pregnancy hypertension. Williams obstetrics. 23rd ed. New York: McGraw Hill;2009.
3.
4.
5.
Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57–70.PubMedCentralPubMedCrossRef
6.
Feig DS, Shah BR, Lipscombe LL, Wu CF, Ray JG, Lowe J, Hwee J, Booth GL. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. 2013;10:e1001425.PubMedCentralPubMedCrossRef
7.
8.
9.
10.
Roberts JM, Balk JL, Bodnar LM, Belizán JM, Bergel E, Martinez A. Nutrient involvement in preeclampsia. J Nutr. 2003;133:1684S–92.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree