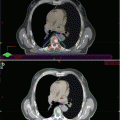
Fig. 6.1
Prostate cancer patient with increased PSA level after radical prostatectomy. (a) 99mTc-MDP planar whole-body bone scan in anterior (left) and posterior (right) views shows multiple increasing tracer uptakes (arrows) suggestive of bone metastases. (b) SPECT/CT from pelvis skeleton (CT upper, fusion lower) is able to better determine the suspicious lesions on whole-body scan (arrows)
Another application of hybrid SPECT/CT imaging is in the assessment of 99mTc-MDP-positive benign bone abnormalities such as spondylopathy, spondylarthrosis, and osteochondrosis. Interesting results revealed by Gnanasegaran and colleagues showed a better diagnostic confidence in differentiating benign from malignant skeletal lesions for interpreters when they used SPECT/CT images compared with SPECT images alone or side-by-side CT correlation [56]. A total of 57 patients with bone metastases from prostate cancer which were interpreted as indeterminate on SPECT images had been enrolled in a study, performed by Römer and colleagues. Among those equivocal lesions which approximately 64 % of them were localized in the spine, more than 90 % were clarified by additional hybrid SPECT/CT modality [57]. Helyar and colleagues retrospectively evaluated 50 skeletal lesions from prostate cancer and found 61 % versus 8 % of equivocal lesions on planar/SPECT and SPECT/CT, respectively. They concluded that in comparison with SPECT or planar bone scintigraphy, SPECT/CT is more accurate with better diagnostic confidence in assessment of osseous metastases from prostate cancer [58]. These data were confirmed in another study by Ndlovu and colleagues. They recruited 42 patients who had a total of 189 bone metastatic lesions. When compared with SPECT imaging alone, the SPECT/CT significantly reduced the number of indeterminate lesions (31 % vs 9 %, p < 0.0001). It also outperformed SPECT alone with an overall accuracy of 92 % versus 67 % (p < 0.0001) on a lesion-wise basis [59]. In another study, Sharma and colleagues realized that from 49 indeterminate lesions on planar imaging, 48 (96 %) were clarified with SPECT/CT method (p < 0.001) which shows a superiority for latter in correctly differentiate osseous metastases from prostate cancer. They also revealed that the management was changed in 61 % of patients based on SPECT/CT data compared with planar whole-body bone scintigraphy [60]. In a most recent study, Palmedo and colleagues discovered significantly better (p < 0.01) specificity (94 % vs 78 %) and positive predictive value (88 % vs 59 %) in the detection of osseous metastases for SPECT/CT scan compared with 99mTc-MDP bone scintigraphy and SPECT alone. The sensitivity calculated as 97 % versus 93 % and NPV as 97 % versus 95 %. There was an approximately 30 % downstaging for enrolled patients based on SPECT/CT data [61]. The abovementioned findings are supported in a review article by Ghosh who emphasized that SPECT/CT imaging provides higher accuracy and better diagnostic confidence in metastases localization and characterization of solitary or indeterminate skeletal lesions [62]. Indeed, the fact was confirmed by other investigators as well [17, 61, 63–67].
6.3 18F-NaF PET/CT
Conventional whole-body scintigraphy is relatively inexpensive method of imaging and has some other advantages such as desirable sensitivity and widespread availability that makes it the first choice in diagnosing skeletal metastases from different cancers, including the lung, breast, and prostate. However, due to reduced spatial resolution, most notably within the spine, pelvic bones, and calvarium, the application of a high-resolution imaging technique such as hybrid positron-emission tomography/computed tomography (PET/CT) is warranted. More accurate than 99mTc-MDP planar bone scintigraphy or SPECT, the 18F-sodium fluoride (18F-NaF) PET/CT had been used to assess skeletal metastatic disease (Figs. 6.2 and 6.3) [68].
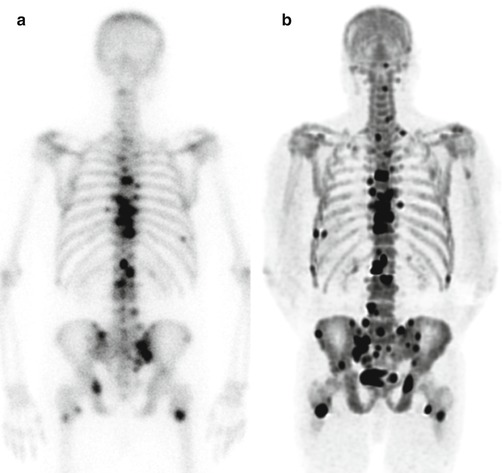
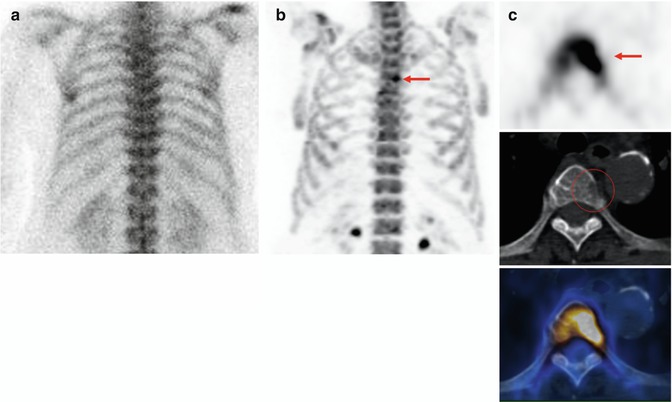

Fig. 6.2
Prostate cancer patient with known bone metastases and increasing PSA level after chemotherapy. (a) 99mTc-MDP whole-body bone scan (posterior view) shows multiple bone metastases on the skeleton. (b) 18F-NaF PET (maximum intensity projection) is able to detect more metastatic lesions with significantly better resolution than convectional bone scan

Fig. 6.3
Staging from a high-risk cancer patient. (a) 99mTc-MDP planar bone scan (posterior view) is unremarkable. (b) 18F-NaF PET (maximum intensity projection) shows suspicious increased tracer uptake on a thoracic spine. (c) 18F-NaF PET/CT (transaxial images): intensive 18F-NaF uptake on a thoracic spine (upper, arrow) with corresponding cortical destruction on CT (Mid), fusion image (lower) confirms the findings on PET and CT
As a positron-emitting radiopharmaceutical, the 18F-NaF had been firstly utilized to image osseous structures more than four decades ago. However, the application was limited due to its high energy of 511 keV which was not optimal for gamma cameras and short half-life of only 110 min. By the introduction of 99mTc-bisphosphonates with desirable peak photon energy, the latter replaced completely for bone imaging. Then, the application of new modality had extended dramatically when its accuracy improved by development of SPECT and SPECT/CT scans later [69]. Nonetheless, the use of PET scanners which are perfectly capable of detecting photons with 511 keV energy is markedly increased in the last decade, which, in turn, introduced 18F agents again for research as well as in clinical studies.
The skeletal uptake of 18F-NaF and 99mTc-MDP is in almost the same way. The agents chemisorb to hydroxyapatite, with the production of fluoroapatite and a hydroxyl group [OH−]:
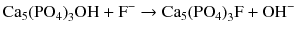
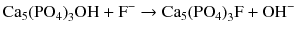
This way, the radiotracers attach to the areas of new formation and mainly represent osteoblastic activity [27, 70].
18F-NaF has a first-pass clearance of approximately 100 %[71]. The bone structures take up to the half of the dose and another 30 % diffuse within the red blood cells. The remaining amount will be excreting by the kidneys within 6 h of injection [72]. Accordingly, the patients that undergo 18F-NaF PET/CT should be perfectly hydrated by drinking more than half a liter of water before and after imaging and more frequently empty the bladder to hasten radiotracer excretion from the body and diminish radiation exposure to urinary bladder as the target organ [72, 73].
The recommended adult dose of 18F-NaF is 40–100 μCi/kg (1.5–3.7 MBq/kg) or a maximum of 10 mCi (370 MBq) for oncologic PET/CT imaging [73]. It is usually 20–25 mCi (740–925 MBq) for 99mTc-MDP [28]. The effective radiation dose is 0.021 mrem and 0.089 mrem per mCi of 99mTc-MDP and 18F-NaF, respectively. However, due to less injected dose and shorter half-life of 18F-NaF (110 min vs 6 h), the total actual radiation absorbed dose is quite similar [72–74].
The indications for 18F-NaF PET/CT are resembling to 99mTc-MDP whole-body bone scan and include the assessment of primary and secondary bone malignancies, evaluation of equivocal findings of other imaging or lab modalities, as well as determination of response to therapy [17].
In a study by Langsteger and colleagues on 22 patients with prostate cancer who underwent 18F-NaF PET/CT, the modality was calculated to be 91 % sensitive, 83 % specific, and 88 % accurate in evaluation of osseous metastases [75].
Considering high sensitivity of 18F-NaF PET to detect osseous lesions, however, interpreting all focal uptakes as metastasis causes an increased number of false-positive reports and reduced specificity of the modality. In fact, an integrated hybrid PET/CT system guides the interpreters to differentiate benign from malignant lesions. This is specifically an important issue in the spinal lesions in most often old prostate cancer patients who are bearing extensive degenerative changes. Therefore, any abnormal focal uptake which is not correlated to joint surface or end plate should be considered as suspicious [76]. Similar to PET/CT scans with other radiopharmaceuticals, a shortcoming of 18F-NaF PET/CT is the inability of tiny lesions to provoke a blastic reaction with resultant tracer uptake, particularly within the spine [77, 78].
Similar to conventional whole-body bone scintigraphy, the interpreters should be aware of “flare phenomenon” when reviewing 18F-NaF PET/CT images for skeletal metastases from prostate cancer [79].
18F-NaF PET/CT scan has the potential for monitoring response to treatment in patients with osseous metastatic disease (Fig. 6.4). Hillner and colleagues released data based on the American National Oncologic PET Registry (NOPR) which demonstrated the impact of 18F-NaF PET/CT on management of these patients. The total of 2217 patients with 2839 imaging studies (68 % prostate, 17 % breast, 6 % lung, 8 % other cancers) was evaluated. 18F-NaF PET/CT caused alteration in 40 % of the treatments, and the impact was particularly high in patients with evidence of progressive skeletal metastatic disease [80].
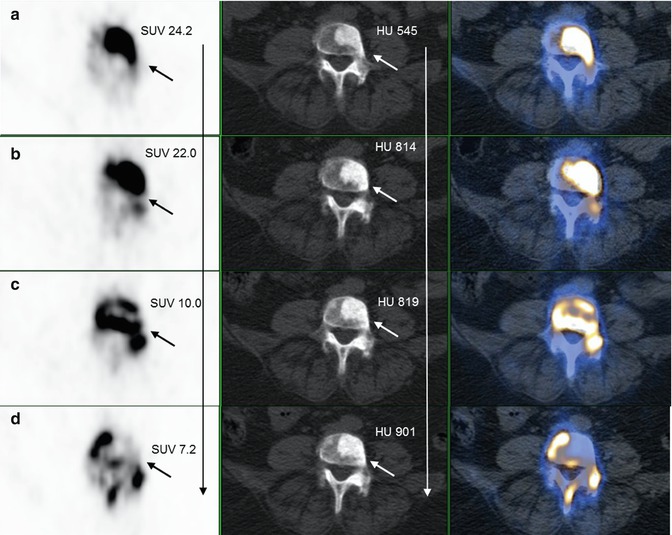

Fig. 6.4
18F-NaF PET/CT (transaxial views); treatment monitoring of a prostate cancer patient with bone metastases before and after therapy. (a) PET image (left) shows a markedly increased 18 F-NaF uptake (left upper row, arrow) corresponding with a sclerotic lesion (mid upper row, arrow) on fourth lumbar spine on CT; fusion PET/CT (right). (b–d) Follow-up 18F-NaF PET/CT shows decreasing pattern of 18F-NaF uptake on PET (left, arrows); however with increasing in Hounsfield unit (HU) of the sclerotic lesion (mid, arrows); clearly shows higher impact of functional (PET) over anatomical (CT) imaging for therapy monitoring
As a fact, the sooner recognition of bone metastases in prostate cancer patients, the better planning for successful therapy as well as evaluation of prognosis (Fig. 6.5). Recently, Apolo and colleagues introduced 18F-NaF PET/CT as a tool with prognostic values. They reviewed 60 patients with bone metastases from prostate cancer and discovered a significant correlation between overall survival and changes of SUV on 6-month follow-up images (p = 0.018) as well as the number of metastases on primary examination (p = 0.017) [81]. However, regarding few investigations, more studies are still needed to establish the prognostic role for 18F-NaF PET/CT in patients with prostate cancer and skeletal metastases.
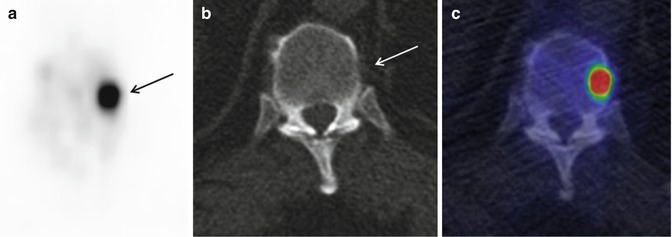

Fig. 6.5
18F-NaF PET/CT in staging of a prostate cancer patient. (a) 18F-NaF PET image (transaxial) shows a focal increased 18 F-NaF uptake (arrow) in a thoracic spine. (b) CT (transaxial) is unremarkable regarding bone metastases. (c) Fusion PET/CT image (transaxial) confirms the findings of PET and CT which are suggestive of early bone metastases without any morphological changes detected by 18F-NaF PET
6.4 Conventional Bone Scintigraphy Versus 18F-NaF PET/CT
There are pros and cons for 18F-NaF PET/CT, compared with conventional 99mTc-MDP whole-body bone scintigraphy. The former has less binding to plasma proteins which cause much more availability with quicker plasma clearance; higher uptake rate by osseous cells and target-to-background ratio with resultant double concentration in metastatic lesions, as well as better imaging quality with better spatial resolution (4–5 versus 10–15 mm); earlier imaging after radiotracer administration (0.5–1 versus 2–3 h); and greater sensitivity [74, 82–84]. Also, some studies reported that 18F-NaF PET/CT is able to image both blastic and lytic skeletal metastatic lesions (Fig. 6.6) [85].
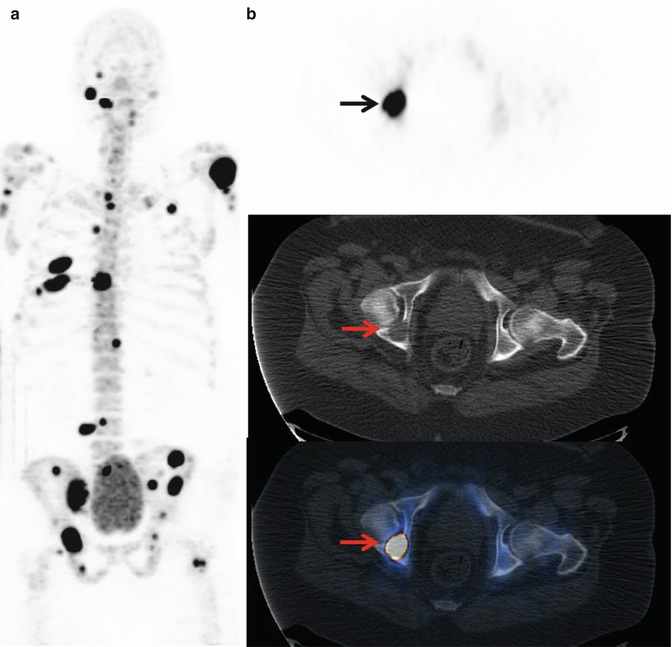

Fig. 6.6
18F-NaF PET/CT in primary staging of a prostate cancer patient with highly increased PSA level. (a) 18F-NaF PET (maximum intensity projection) shows multiple metastatic lesions in the skeleton. (b) 18F-NaF PET/CT (transaxial images): intensive 18F-NaF uptake on the right acetabulum (upper, arrow) with corresponding osteolytic lesion on CT (Mid, arrow). Fusion image (lower, arrow) shows that 18F-NaF PET/CT is also able to detect osteolytic bone metastases
Despite the aforementioned advantages, PET/CT scanners are less available than gamma cameras, and the study is more expensive. On the other hand, the insurance reimbursement is more organized for conventional nuclear medicine imaging compared with 18F-NaF PET/CT. While the 99mTc is produced readily by generator, the production of 18F needs a cyclotron which makes an extra cost. Overall, the PET/CT cost-effectiveness needs to be further investigated in the future studies [17].
Generally, the sensitivity and specificity to detect bone metastases from prostate cancer are higher for 18F-NaF PET/CT than whole-body bone scintigraphy. Even-Sapir and colleagues reviewed 23 patients and calculated a patient-based sensitivity of 100 % and 70 % and specificity of 100 % and 57 % for 18F-NaF PET/CT and planar whole-body bone scintigraphy, respectively. This superiority approved on a lesion-based analysis as well. They also realized that compared with SPECT imaging, PET/CT shows significantly better sensitivity and specificity (p < 0.05) [17]. Also, Evangelista and colleagues revealed in their review an article that the 18F-NaF PET/CT has the best median sensitivity (with a range of 81–100 %) among the nuclear medicine modalities in evaluation of bone metastases from prostate cancer [83].
Bombardieri and colleagues revealed that 18F-NaF PET/CT is more sensitive and superior to whole-body bone scan in evaluation of osseous metastases from prostate cancer, most likely due to fast uptake of radiotracer by bone lesions and quick excretion by urinary system, leading to higher target-to-background ratio. Also, some other investigators had almost similar conclusion [17, 67, 75, 86–89].
Furthermore, the 18F-NaF PET/CT depicts more number of lesions and earlier in the course of disease when compared with conventional bone scintigraphy [81].
The superiority of 18F-NaF PET/CT over conventional bone scan in sensitivity, NPV, and accuracy was the conclusion of a study by Poulsen and colleagues, in which they evaluated 50 patients with 526 metastatic bone lesions from prostate cancer. With a sensitivity of 93 %, however, the specificity was reported as low as 54 %, most likely due to false-positive lesions from inflammatory or degenerative process among the enrolled old-age patients. The sensitivity, specificity, accuracy, and positive and negative predictive values were reported as 93 % vs 51 %, 54 % vs 82 %, 81 % vs 61 %, 82 % vs 86 %, and 78 % vs 43 % for 18F-NaF PET/CT versus 99mTc-MDP whole-body scintigraphy, respectively [70].
18F-NaF PET/CT is more accurate for evaluation of response to treatment among patients with prostate cancer osseous metastatic disease [90].
Jadvar and colleagues investigated 37 patients with history of localized prostate cancer, followed by a definitive therapy such as radiation or radical prostatectomy, who presented with evidence of PSA biochemical recurrence but negative conventional studies. They demonstrated that the 18F-NaF PET/CT is capable of detecting occult skeletal metastatic lesion in lower serum PSA levels than conventional imaging [91].
When compared with conventional whole-body bone scan, semiquantitative evaluation of skeletal lesions with calculation of standardized uptake value (SUV) is a unique advantage of 18F-NaF PET/CT. This is actually important in the spine as the most frequent site of both 18F-NaF-positive degenerative changes and metastases from prostate cancer [92]. Muzahir and colleagues performed a pilot investigation in which the results showed a significantly lower SUVmax for degenerative (<12) versus metastases from castrate-resistant prostate cancer (>50) in the spine (p < 0.001). They enrolled 17 patients with history of castrate-resistant prostate cancer and 65 metastatic as well as 56 degenerative 18F-NaF-positive lesions, differentiated according to low-dose CT scan. The range of SUVmax was calculated as 11–188 (mean of 160) for metastatic versus 3–50 (mean of 6.2) for degenerative lesions. The authors concluded that semiquantitative measurements can play a complementary role for qualitative analysis in order to better differentiate osseous metastatic from degenerative lesions [93].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree