Preliminary therapeutic successes have prompted a new wave of clinical trials enrolling patients with myelodysplastic syndromes (MDS), using compounds with a broad range of potential mechanisms of action. This article discusses several of the agents currently in development for MDS, reviewing clinical trial data related to five classes of novel therapeutics: clofarabine, a halogenated purine nucleoside analog; ezatiostat (TLK199), a glutathione analog that indirectly activates c-Jun kinase; tipifarnib, a farnesyltransferase inhibitor; laromustine (cloretazine), an alkylating agent with a metabolite that inhibits one mechanism of DNA damage repair; and eight drugs that inhibit histone deacetylase. Although MDS are still difficult clinical problems, and most patients with MDS still succumb to disease-related complications within 3 to 5 years of diagnosis, ongoing development of novel agents promises that there will be new treatment options for patients within the next 5 to 10 years.
The myelodysplastic syndromes (MDS), a diverse collection of neoplastic disorders characterized by marrow failure and a risk of clonal progression, were long considered an unexciting backwater of hematology practice, because of a lack of effective therapies other than supportive care or allogeneic stem cell transplantation. However, United States Food and Drug Administration (FDA) approval of three drugs for MDS-related indications (azacitidine [Vidaza] in 2004; lenalidomide [Revlimid] in 2005; and decitabine [Dacogen] in 2006), followed by a 2007 report that azacitidine treatment improves median survival of higher-risk patients with MDS by 9 months, began to change the biomedical community’s perceptions of these difficult disorders.
No longer are MDS viewed solely with a sense of therapeutic nihilism. Today, more than 300 clinical trials, using more than 50 experimental compounds, are actively recruiting patients with MDS to improve patients’ quality of life and alter the natural history of disease ( Box 1 ). Other studies of novel compounds and combinations of drugs have recently completed enrollment and results will soon be reported, while additional biologically interesting molecules are in late preclinical stages of development.
Trials using FDA-approved agents for MDS-related indications, either alone or in combination
Azacitidine
Monotherapy (including an oral formulation): four trials
With histone deacetylases (HDAC) inhibitors (vorinostat, valproate, panobinostat, belinostat, MS-275): six trials
With lenalidomide: two trials
With thalidomide: one trial
With chemotherapy: one trial
With bortezomib: one trial
Decitabine
Monotherapy (including an oral formulation): four trials
With HDAC inhibitors: two trials
With gemtuzumab: one trial
With clofarabine: one trial
With lenalidomide: one trial
With arsenic and vitamin C: one trial
With tretinoin: one trial
With cytotoxic chemotherapy: one trial
Lenalidomide
Monotherapy: eight trials
With or without epoetin alfa: one trial
With romiplostim: one trial
With stem cell factor: one trial
With vaccine: three trials
Iron chelation with deferasirox or deferoxamine: seven trials
Trials using drugs that are FDA-approved for conditions other than MDS
Alemtuzumab + cyclosporine A: one trial
Arsenic trioxide + vitamin C: one trial
ATG + cyclosporine A: two trials
Bendamustine: one trial
Bevacizumab: one trial
Bexarotene: one trial
Bortezomib, alone or in combinations other than with azacitidine: three trials
Calcitriol + dexamethasone: one trial
Clofarabine
Monotherapy: six trials
With decitabine or cytarabine: two trials
Cytotoxic chemotherapy: two trials
With HDAC inhibitor: two trials
With tipifarnib or gemtuzumab: one trial
Dasatinib: one trial
Eltrombopag: two trials
Erythropoiesis-stimulating agents (monotherapy): four trials
Everolimus (RAD001)
Monotherapy: one trial
With PKC412 (protein kinase C inhibitor): one trial
Romiplostim
Monotherapy: two trials
With lenalidomide: one trial
Sorafenib
Monotherapy: two trials
With chemotherapy: two trials
With clofarabine: one trial
With vorinostat: one trial
Vorinostat monotherapy: one trial
Trials using HDAC inhibitor monotherapy (other than vorinostat)
Belinostat: one trial
JNJ-26481585: one trial
NVP-LBH 589: three trials
SNDX-275 (MS-275): two trials
Trials using agents not yet FDA approved for any condition
ABT-888 [PARP inhibitor] + chemotherapy: one trial
Afibercept [anti-VEGF agent]: one trial
ARRY-614 [multikinase inhibitor]: one trial
AT9283 [Aurora kinase inhibitor]: one trial
Cediranib (AZD2171) [VEGF inhibitor]: one trial
CP-4055 [cytarabine prodrug]: one trial
DB-67 (AR-67) [camptothecin]: two trials
Ezatiostat (TLK199) [glutathione S1 analog]: one trial
Gimatecan (ST1481) [topoisomerase inhibitor]: one trial
GTI-2040 [ribonucleotide reductase antisense oligonucleotide]: one trial
HuM195 [humanized anti-CD33 antibody]: two trials
IMC-A12 [IGF-1R monoclonal antibody] + temsirolimus: one trial
INCB018424 [JAK2 inhibitor]: one trial
Laromustine (cloretazine) [novel alkylator] + cytarabine: one trial
MLN4924 [Nedd8 activating enzyme inhibitor]: one trial
MLN8237 [Aurora kinase inhibitor]: one trial
MultiStemA [manufactured stem cell product]: one trial
NOV-002 [chemoprotectant]: one trial
ON 01910.Na [cyclin D1 inhibitor]: five trials
PF-04449913 [Hedgehog inhibitor]: one trial
Sapacitabine (CYC682) [nucleoside analog]: one trial
SB1518 [JAK2 inhibitor]: one trial
SNS-595 [topoisomerase II inihibitor and DNA damage causing agent]: one trial
STA-9090 [Hsp90 inhibitor]: one trial
TAK-901 [Aurora kinase inhibitor]: one trial
Tipifarnib [farnesyltransferase inhibitor]: one trial
UCN-01 [staurosporine analog] + perifosine [modulator of membrane permeability]: one trial
Vaccine approaches (nontransplant): 11 trials
Data from www.clinicaltrials.gov . Accessed August 27, 2009.
Of 432 trials returned by a search for “Recruiting,” “Investigational,” and “MDS,” 91 trials were excluded because they were recruiting only patients with diseases other than with MDS, or were testing supportive therapies not designed to alter blood counts or the underlying condition (eg, massage therapy, bisphosphonates, or antiemetics). Additionally, 96 trials studying various aspects of stem cell transplantation (eg, conditioning regimens, stem cell product manipulation, and maintenance approaches) are not listed here.
Despite a new sense of optimism and an exciting flurry of investigative activity, the therapeutic gains achieved have been modest. Even the encouraging 9-month median increase in survival with azacitidine treatment still represents less than 5% of the 15.4-year life expectancy for a 65-year-old American man without MDS. Furthermore, the clinical, cytogenetic, and molecular heterogeneity of MDS, and the high proportion of patients with these syndromes who are elderly and frail, remain challenging obstacles to rapid clinical trial accrual and successful drug development. Much about MDS pathobiology remains mysterious, which impedes development of targeted therapies; even the precise mechanisms of action of the three FDA-approved medications are currently unclear.
This article discusses five different types of agents currently undergoing clinical trials in MDS: (1) histone deacetylase inhibitors; (2) the purine nucleoside clofarabine; (3) the glutathione S-transferase analog ezatiostat (TLK199); (4) the novel alkylating agent laromustine (cloretazine); and (5) the farnesyltransferase inhibitor tipifarnib. This sample is by no means comprehensive, but these classes of agents are representative of contemporary developmental therapeutic approaches to MDS. Most of these medications have also been studied in patients with acute myeloid leukemia (AML), and AML data are discussed later. Trial results in patients with myeloproliferative neoplasms are beyond the scope of the present discussion, however, because the clinical problems encountered in patients with myeloproliferative neoplasms, such as primary myelofibrosis (eg, splenomegaly, constitutional symptoms, and thrombohemorragic events), are less common in MDS.
Histone deacetylase inhibitors
Histone Modification and Biologic Effects
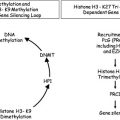
Octamers of histone proteins are the chief protein component of chromatin. Histones play an important structural role in the eukaryotic nucleus, binding DNA with a similar topology to a spool wound with thread. Yet, histones are not merely inert DNA packaging material: they are dynamic proteins, undergoing numerous posttranslational modifications, including methylation, acetylation, ubiquitination, phosphorylation, and SUMOylation (modifications collectively called the “histone code”), which can result in changes in the accessibility of associated DNA to transcription, and alter interactions of the nucleosome with chromatin-associated proteins.
One of the most important histone posttranslational modifications is acetylation of lysine residues of histone subunit H3. Acetylation at lysine residues 9 or 14 (ie, K9 and K14) can result in transcriptional activation of the DNA associated with the acetylated histone; such acetylation is catalyzed by histone lysine acetyltransferases (KATs). In contrast, acetyl groups are removed by histone deacetylases (HDACs), of which there are four classes with at least 11 enzyme members, with both class-specific and enzyme-specific biologic effects.
Although there is no direct evidence yet of specific regions of pathologic histone deacetylation in MDS, clinical success in MDS with the methylation modifiers azacitidine and decitabine led investigators to consider the therapeutic possibilities of alteration of HDAC activity also, because histone acetylation and DNA hypomethylation are coupled and alter gene expression in a complementary manner. In a perhaps naive view (but potentially a correct one, at least in part), inhibition of HDACs could shift the balance in favor of KAT enzyme activity, resulting in increased histone acetylation and consequent increased transcriptional activity of tumor suppressor genes, thereby eliminating neoplastic cells that are dependent on transcriptional silencing of tumor suppressors.
The reality is almost certainly more complex. Despite their name, HDACs have a number of nonhistone protein targets for deacetylation, including nuclear and cytoplasmic proteins that regulate processes altered in neoplasia, such as apoptosis, differentiation, and cell proliferation. As for azacitidine and decitabine, which inhibit DNA methyltransferases and act by epigenetic mechanisms (See the article by Jean-Pierre Issa elsewhere in this issue for further exploration of this topic.), but also damage DNA in a fashion similar to traditional cytotoxic drugs, any therapeutic effects of HDAC inhibitors should not be attributed to reawakening silenced tumor suppressors without convincing evidence. In addition, transcriptional profiling experiments indicate that expression of only a small proportion of genes (<5%) is altered by HDAC inhibition, raising further questions about whether the most likely clinical effect of this class of drugs is a result of changing gene expression, or something else.
HDAC Inhibitors as a Drug Class
Pharmaceutical companies have developed a number of HDAC inhibitors with varying potency and chemical structural class ( Table 1 ), and dozens of clinical trials with these agents are currently enrolling patients with hematologic malignancies or solid tumors. Some compounds inhibit specific HDAC enzymes, such as entinostat (SNDX-275/MS-275) or MGCD0103 (MethylGene), which selectively inhibit only class I HDACs (ie, HDACs 1, 2, 3, and 8). Other agents, such as panobinostat (NVP-LBH589), inhibit HDACs more broadly. It is not clear whether class specificity is a desirable property of an HDAC inhibitor, but it is possible that inhibition of certain classes of enzyme and not others could alter the repertoire of cytoplasmic proteins modified, and effect the adverse event profile. In clinical trials, most HDAC inhibitors have been associated with gastrointestinal side effects, severe fatigue, and thrombocytopenia as the most common treatment-related adverse events. QTc monitoring is also recommended, although clinically significant QTc prolongation has proved uncommon.
| HDAC Inhibitor | Sponsor | Chemical Class | Data in MDS or AML? |
|---|---|---|---|
| Valproic acid a | Multiple suppliers | Branched short-chain fatty acid | Yes, Phase II |
| Sodium phenylbutyrate and phenylacetate a | Ucyclyd | Aromatic short-chain fatty acid | Yes, Phase II |
| AN-9/pivaloyloxymethyl butyrate b | Titan | Aliphatic short-chain fatty acid | No |
| FK228/FR901228/depsipeptide/romidepsin | Gloucester (now part of Celgene) | Tetrapeptide | Yes, Phase II |
| ITF2357/givinostat | Italfarmaco | Hydroxamic acid | No |
| JNJ-26481585 | Centocor OrthoBiotech | Hydroxamic acid | No |
| NVP-LBH589/panobinostat | Novartis | Hydroxamic acid | Yes, Phase I/II |
| NVP-LAQ824/dacinostat | Novartis | Hydroxamic acid | No |
| PCI-24781 | Pharmacyclics | Hydroxamic acid | No |
| PXD101/belinostat | Curagen and Topotarget A/S | Hydroxamic acid | Yes, Phase II |
| SAHA/vorinostat a | Merck | Hydroxamic acid | Yes, Phase II |
| CI-994/N-acetyldinaline | Pfizer | Benzamide | No |
| MGCD0103 c | MethylGene | Benzamide | Yes, Phase I/II |
| SNDX-275/MS-275/entinostat | Syndax | Benzamide | Yes, Phase I; Phase III ongoing |
a As of this writing, SAHA/vorinostat and romidepsin are the only HDAC inhibitors to have achieved Food and Drug Administration approval for treatment of a neoplasm: cutaneous T-cell lymphoma. Sodium phenylbutyrate and sodium phenylacetate (Buphenyl and Ammonul) are marketed as orphan drugs, but for hyperammonemia related to urea cycle disorders rather than neoplasia. Valproic acid is used as an anticonvulsant and mood-stabilizing agent.
b Development discontinued because of adverse events.
c Partial clinical hold since August 2008 because of pericardial effusion adverse event.
Vorinostat (suberoylanilide hydroxamic acid, Zolinza) was the first, HDAC inhibitor to receive formal regulatory approval; the FDA licensed vorinostat in 2006 for treatment of patients with cutaneous T-cell lymphoma. Romidepsin was approved for the same indication in November 2009. Investigators have also “repurposed” as HDAC inhibitors several drugs that were originally developed and approved for other indications, after those drugs were serendipitously found during screening programs to have HDAC inhibitory activity. Examples include valproic acid, an anticonvulsant, and sodium phenylbutyrate, an agent used to treat patients with congenital urea cycle disorders.
The drug development strategy pursued by different HDAC inhibitor sponsors has varied, depending on preclinical signals and marketing considerations. Some developmental programs are focusing on solid tumors or, given the success of vorinostat in cutaneous T-cell lymphoma, on lymphoproliferative disorders. Other sponsors, having noted the success of azacitidine and decitabine for MDS, are performing trials in the MDS and AML settings (most trials to date have enrolled both higher-risk MDS and AML patients). Agents for which there are at least preliminary data in MDS and AML include entinostat; belinostat (PXD101); romidepsin (FK228/FR901229/depsipepide); panobinostat; MGCD0103; valproic acid; and sodium phenylbutyrate.
Entinostat (SNDX-275/MS-275)
A Phase I study of oral entinostat in 38 patients with acute leukemia found that the maximum tolerated dose (MTD) was 8 mg/m 2 administered once weekly for 4 weeks every 6 weeks. Adverse events included somnolence, unsteady gait (the dose-limiting toxicity [DLT]), and severe fatigue; gastrointestinal side effects, hypoalbuminemia, and hypocalcemia were also observed. No objective clinical responses were seen, however, indicating that entinostat has limited activity as monotherapy in leukemia.
In May 2009, the Eastern Cooperative Oncology Group (ECOG) suspended accrual to intergroup Phase III protocol ECOG E1905 after the study met its initial target enrollment of at least 152 patients. In the E1905 protocol, patients with higher-risk MDS, chronic myelomonocytic leukemia (CMML) with a white blood count less than 12 × 10 9 /L, or AML with trilineage dysplasia and white blood count less than 30 × 10 9 /L were randomized to receive either azacitidine at a nonstandard dose of 50 mg/m 2 subcutaneously daily for 10 days every 28 days, or subcutaneous azacitidine using that same nonstandard schedule plus oral entinostat, 4 mg/m 2 , on days 3 and 10 of each treatment cycle. Because single-agent activity of HDAC inhibitors in MDS may be limited, this study will answer the important question of whether HDAC inhibitors might instead augment response to hypomethylating agents. In a Phase I study of 27 patients with MDS, AML, or CMML that used the same azacitidine-entinostat combination used in E1905, 12 patients responded, including two complete remissions. Results of the E1905 study are expected by 2010.
Belinostat (PXD101)
After a Phase I study in patients with solid tumors identified the MTD of belinostat as 1000 mg/m 2 administered intravenously once daily over 30 minutes for 5 consecutive days every 21 days, another Phase I belinostat study enrolled 16 patients with advanced hematologic malignancies (none had MDS or leukemia). This study identified fatigue and neurotoxicity as the most common treatment-related adverse events; no formal tumor responses were seen, but two patients with multiple myeloma developed transient tumor lysis and associated grade 4 renal insufficiency.
The National Cancer Institute–sponsored Phase 2 Consortium recently suspended accrual to a Phase II study of belinostat that was enrolling patients with MDS-associated cytopenias whose disease had failed to respond to azacitidine. There were no complete or partial responses meeting International Working Group MDS response criteria. As with entinostat, belinostat seems to have limited efficacy as a single agent in MDS; however, it is still possible that these agents might have a role in combination therapy.
Romidepsin (FK228/depsipeptide)
In a Phase II study of 12 patients, 9 with AML and 3 with MDS, romidepsin was administered intravenously at a dose of 18 mg/m 2 on day 1 and day 5 every 21 days, the MTD that had been identified in solid tumor studies with romidepsin. The investigators observed no consistent changes in histone acetylation in primary cells after romidepsin use. One patient with AML experienced a complete response. Recurrent adverse events included febrile neutropenia, thrombocytopenia, nausea, and asymptomatic hypophosphatemia.
The authors of the report of this Phase II study, in a commendably honest admission (particularly in view of the lamentable contemporary tendency to describe any nonlethal regimen as “well-tolerated,” and all regimens as “worthy of further study” regardless of response rate) concluded, “depsipeptide monotherapy has limited clinical activity in unselected AML/MDS patients.” On November 5, 2009, romidepsin was approved by the US FDA for the treatment of cutaneous T cell lymphoma.
Panobinostat (NVP-LBH589)
A Phase I/II study of oral panobinostat in advanced hematologic malignancies identified 60 mg administered once weekly as the MTD in patients with AML, with the DLT being fatigue (drug-associated grade 4 thrombocytopenia and neutropenia were also common). Among 26 evaluable patients at the time of a preliminary report of that study (65 patients with AML were enrolled), there were two complete responses. A case report described a patient with AML who experienced tumor lysis syndrome during panobinostat monotherapy. Panobinostat data in patients with MDS have not yet been reported. As for some other HDAC inhibitors, thrombocytopenia may make it difficult to combine panobinostat with azacitidine or decitabine in MDS.
Valproic acid
Valproic acid specifically inhibits HDAC2. In most studies of valproic acid used as a HDAC inhibitor, the dose has been adjusted to achieve serum concentrations of 50 to 100 μg/mL, equivalent to 0.347 to 0.694 mM, levels at which HDAC inhibition is present (concentrations of >0.25 mM increase histone H4 acetylation), but not maximal (HDAC inhibition is “massive” at concentrations of 2 mM). The main limitation of valproic acid as an antineoplastic agent is that in doses required to attain sustained levels associated with robust HDAC inhibition, neurotoxicity can be problematic. For instance, in a Phase II study of MDS-CMML using valproic acid plus 13- cis -retinoic acid and 1,25-dihydroxyvitamin D, 8 (42%) of 19 enrolled patients had to discontinue therapy because of adverse events, whereas only 3 patients (16%) experienced a measurable response.
In a Phase II study of 75 patients with MDS, AML, or CMML treated with valproic acid (initially as monotherapy in 66 patients, followed by all- trans -retinoic acid as a potential differentiating agent in nonresponders), 1 patient achieved a complete response, 1 achieved a partial response, and 12 patients achieved a major erythroid response (19% overall response rate). Overall, the adverse events with doses used in this study were mild, with four patients developing tremor and three patients experiencing reversible thrombocytopenia. The specific contribution of all- trans -retinoic acid to the observed responses is unclear. A 26-patient Phase II trial of the same drug combination in patients with poor-risk leukemia resulted in no complete responses.
Valproic acid has also been used in combination with decitabine and azacitidine. In a Phase I/II study of 54 patients with MDS (N = 10) or leukemia, 8 patients (15%) responded to a decitabine and valproic acid combination for a median of 7 months. These responses were associated with an increase in histone H3 and H4 acetylation. The contribution of the decitabine alone to the responses, however, is unclear. A randomized study of 67 evaluable patients with MDS, AML, or CMML attempted to isolate the effect of the valproic acid by comparing decitabine at a dose of 20 mg/m 2 daily for 5 consecutive days every 28 days with the same decitabine regimen plus valproic acid. There was no significant difference in the response rate in the decitabine monotherapy arm compared with the combined therapy arm, and neurotoxicity was common in the arm that included valproic acid. In a Phase II trial of the combination of azacitidine and valproic acid, which enrolled 62 patients with MDS or CMML, the response rate observed was not higher than the rate expected with azacitidine alone.
Sodium phenylbutyrate
Sodium phenylacetate has HDAC inhibitory properties, but is rarely used clinically because of its musky, foul odor (it can be isolated from the defensive glands of the aptly named stinkpot turtle). Sodium phenylbutyrate is a less offensive prodrug of sodium phenylacetate.
A pilot study enrolled 23 patients with AML or MDS, and administered sodium phenylbutyrate by continuous intravenous infusion for 7 days out of 14, or 21 consecutive days out of 28. Two patients achieved hematologic improvement, and the DLT was somnolence. In a small Phase II study that enrolled eight patients with AML and two with MDS, standard 7-day subcutaneous injections of azacitidine, followed by 5 days of sodium phenylbutyrate given intravenously at a dose of 200 mg/kg/d, led to partial response or stable disease in five patients. Most (>80%) patients experienced somnolence and injection site reactions. Histone H4 reacetylation did not correlate with clinical response.
Because of the inconvenient administration schedules required to achieve biologically effective serum concentrations, sodium phenylbutyrate is not being developed further as an HDAC inhibitor in MDS.
MGCD0103
A Phase I study of oral MGCD0103 enrolled 29 patients with either MDS (N = 7) or AML (N = 22) and determined that the MTD for this agent is 60 mg/m 2 administered three times weekly. Three patients (10%) in this Phase I study experienced reduction in marrow blasts to less than 5%, whereas DLTs included fatigue and gastrointestinal effects (nausea, vomiting, or diarrhea). Drug-induced histone acetylation was detected, and was dose dependent.
Subsequently, a Phase I/II study enrolled 37 evaluable patients with MDS (N = 6) or AML (N = 31) and combined a standard azacitidine schedule with MGCD0103. When used in combination, the MTD of MGCD0103 was found to be 90 mg/m 2 administered three times weekly, slightly higher than the MTD determined in the monotherapy Phase I trial. Eleven patients (30%) experienced a response, including nine complete responses, with or without hematopoietic recovery. This complete response rate is somewhat higher than might be expected with azacitidine alone, but numbers of treated patients are small.
A follow-up randomized study of azacitidine with or without MGCD0103 was planned, but unfortunately an adverse event of hemodynamically significant pericardial effusion led the FDA to put a partial clinical hold on the development of this agent in August 2008, preventing accrual of new patients to open clinical studies. Shortly thereafter, the drug’s sponsor, Celgene, who had acquired the agent when purchasing Pharmion in March 2008, terminated a collaborative agreement with MethylGene, so that MethylGene reacquired the rights to MGCD0103. As of this writing, the FDA hold is still in effect, and the developmental future of this compound is uncertain.
Vorinostat
The reason that vorinostat and other HDAC inhibitors are clinically active in T-cell lymphoma is unclear. It is possible that the clinical effects of vorinostat may not result from HDAC activity, but may be a consequence of acetylation of cytoplasmic proteins instead.
In a Phase I study of oral vorinostat enrolling 31 patients with AML and 3 with MDS, the MTD was determined to be 200 mg twice daily or 250 mg three times daily. DLTs included fatigue and gastrointestinal side effects; grade 3 or 4 thrombocytopenia was seen in 12% of patients. Four complete responses were seen, including two with incomplete hematopoietic recovery, all in patients with AML.
There is also preliminary experience with vorinostat used in combination with decitabine, albeit exclusively in AML. In a Phase I study of sequential dosing of 5-day intravenous decitabine schedules and 14-day oral vorinostat in 30 evaluable patients with relapsed or refractory leukemia, one patient experienced a brief complete remission (5 weeks), and four patients enjoyed a reduction in bone marrow blast proportion.
Summary: HDAC Inhibitors
The HDAC inhibitors seem to have limited single-agent activity in MDS. Combination regimens are currently considered more promising. Given the potential of some HDAC inhibitors to cause thrombocytopenia, their use in combination with other cytopenia-inducing agents, such as azacitidine or decitabine, must be approached cautiously, and may ultimately limit the use of this class of drugs.
The sequence of administration of HDAC inhibitors relative to other agents may also be important. For example, in vitro data indicate that administering azacitidine before the HDAC inhibitor entinostat maximizes biologic synergy, whereas administration of entinostat followed by azacitidine is less effective. These schedule questions will also have to be addressed in clinical trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree